Figure 1. Structures of MHC class I molecules and their distinct intracellular assembly modes.
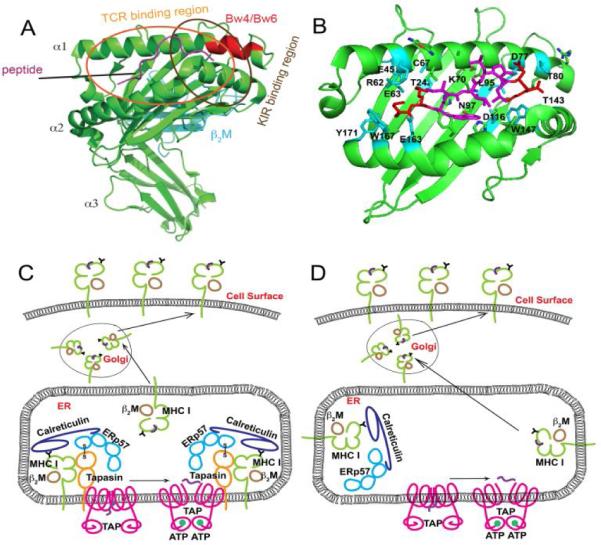
A) Structure of HLA-B*2705 (PDB 4G9D), with heavy chains indicated in green and β2m in cyan. The Bw4/Bw6 epitope region is shown in red. Bound peptide (KRWIILGLNK) is indicated in magenta. Approximate TCR and KIR footprints are specified by ovals. B) Peptide binding groove (α1 and α2 domains) of HLA-B*2705 with bound KRWIILGLNK. Several polymorphic HLA-B*2705 residues important for peptide binding specificity are indicated as sticks. Polymorphic residues interacting with peptide N- and C-termini are shown in cyan. HLA-B*2705 has cysteine, lysine and asparagine respectively at positions 67, 70 and 97. These residuesare shown to be important in control of AIDS progression. Bound peptide is shown in magenta and red, with terminal residues colored red and the central residues colored magenta. C) Conventional tapasin-dependent assembly of HLA-B molecules such as HLA-B*4402 and HLA-B*5701. D) Tapasin-independent assembly of HLA-B molecules such as HLA-B*3501 and HLA-B*4405.
