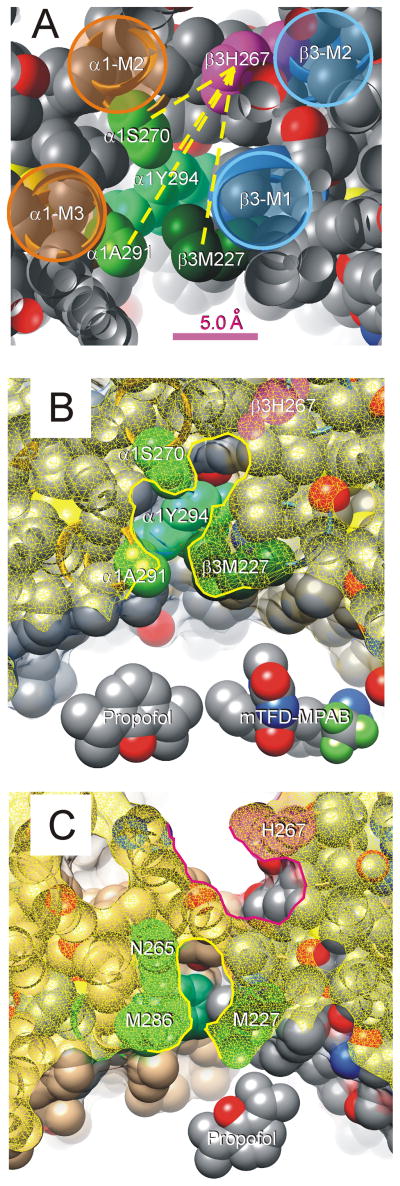
Figure 7. β3H267 and Other α+/β-Anesthetic Contact Residues Line a Contiguous Pocket.
A: A portion of our α1β3γ2L structural homology model is shown with peptide backbone as ribbons and side-chains depicted as spherical shells (hydrogens are hidden). The view is from the extracellular space, off-axis, through a planar cut (atoms cut by this plane appear hollow). The peptide backbones of transmembrane helices are highlighted and labeled. The side-chain of β3H267 is colored magenta, and other side-chains known to contribute to anesthetic binding are shaded in green and labeled. Other side-chain atoms are color coded (gray = carbon, red = oxygen; blue = nitrogen; yellow = sulfur). Some side-chains (β3L223, β3Q224, α1R274, α1M286, and α1D287) were hidden in order to un-roof the cavity that contacts residues of interest. Yellow dotted lines connecting β3H267 to other side-chains represent measured distances in the model, which range from 7.0 Å (to α1S270) to 12.8 Å (to α1A291). B: A view of our homology model similar to that in panel A is shown. The protein surface has been added and is depicted as a translucent film. The “cut plane” is about 1 helical turn (4 Å) more intracellular than that in panel A, and the cut surface shown as yellow mesh. The highlighted border of the cut surface outlines the proposed anesthetic-binding pocket that is lined by β3H267 (magenta) and the other residues that contribute to anesthetic binding (green). Models of propofol and mTFD-MPAB are included for size comparison. C: A “cut” view of the crystallized β3 homomeric receptor structure6. The cut surface is again shown as a yellow mesh. Note that H267 (magenta) forms part of a pocket (highlighted in red) adjacent to the ion channel. Sidechains of P228 and T266 separate the pocket containing H267 from another (highlighted in yellow) that includes other anesthetic photolabeled residues (green) and part of the lipid-protein interface.