Figure 1.
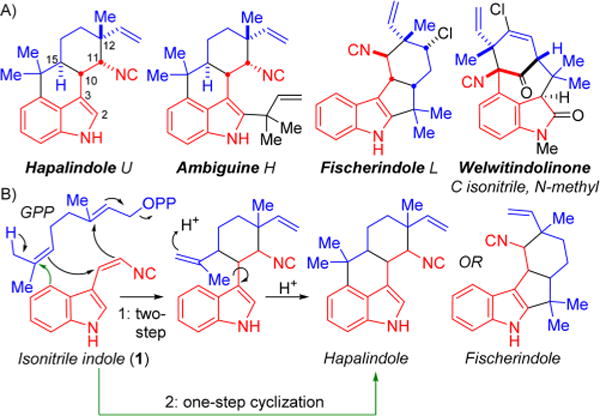
(A) Examples of the four hapalindole alkaloid subgroups with the indole core (red) and monoterpenoid group (blue) highlighted. (B) Current proposed hypotheses for biosynthetic formation of the tri- and tetracyclic cores: Moore proposed a proton-promoted two-step cyclization,15 whereas Carmeli proposed a concerted enzymatic cyclization.18
