Abstract
Purpose
Studies show that deficit syndrome schizophrenia patients, characterized by primary negative symptoms and poor functional outcome, have impairment in specific neural circuits. We assessed whether these same neural circuits are directly linked to functional outcomes across schizophrenia patients.
Methods
T1- and diffusion-weighted MR images were obtained for schizophrenia (n=30) and matched healthy control participants (n=30). Negative symptoms and functional outcome were assessed at baseline and 6-month follow-up. Cortical thickness and tract-wise fractional anisotropy (FA) were compared between groups. To assess relationships of neuroimaging measures with functional outcome, principal component analysis (PCA) was performed on tract-wise FA values and components were entered into a multiple regression model for schizophrenia participants.
Results
Consistent with the literature, schizophrenia participants showed frontotemporal reductions in cortical thickness and tract-wise FA compared to controls. The top two components from PCA explained 71% of the variance in tract-wise FA values. The second component (associated with inferior longitudinal and arcuate fasciculus FA) was significantly correlated with functional outcome (baseline: β=0.54, p=0.03; follow-up: β=0.74, p=0.047); further analysis revealed this effect was mediated by negative symptoms. Post-hoc network analysis revealed increased cortical coupling between right inferior frontal and supramarginal gyri (connected by the arcuate fasciculus) in schizophrenia participants with poorer functional outcome.
Conclusions
Our findings indicate that impairment in the same neural circuitry susceptible in deficit syndrome schizophrenia predicts functional outcome in a continuous manner in schizophrenia participants. This relationship was mediated by negative symptom burden. Our findings provide novel evidence for brain-based biomarkers of longitudinal functional outcome in people with schizophrenia.
Keywords: diffusion tensor imaging, fractional anisotropy, arcuate fasciculus, inferior longitudinal fasciculus, cortical thickness, network analysis
1. Introduction
Schizophrenia is almost certainly a heterogeneous group of disorders for which specific and reliable neurobiological correlates have yet to be identified1,2. Schizophrenia is associated with a high risk of long-term disability and poor functional outcome (Breier A et al., 1991). In people with schizophrenia, more severe negative symptoms and cognitive deficits have independently been associated with poorer functional outcome (Blanchard et al., 2005; Green et al., 2000; Ho et al., 1998; Milev et al., 2005; Rosenheck et al., 2006). However, negative symptoms may mediate the relationship between neurocognition and functioning in schizophrenia (Ventura et al., 2009). Furthermore, negative symptoms predict variance in functional outcome in community dwelling outpatients above and beyond neurocognitive impairment (G Foussias et al., 2011; Foussias et al., 2009).
Inter-regional dysconnectivity and white matter impairment in schizophrenia has been well-established through diffusion tensor imaging (DTI) studies (Kubicki et al., 2007; Wheeler and Voineskos, 2014). Fractional anisotropy (FA) is a validated measure of white matter tract microstructure in diffusion imaging studies and has been associated with neurobiological changes in animal models of schizophrenia such as abnormal expression of myelination genes (Fatemi and Folsom, 2009). In deficit syndrome schizophrenia, characterized by prominent negative symptoms and poor functional outcome (Carpenter et al., 1988; Kirkpatrick B et al., 2001), specific impairments in white matter tract circuitry have been found in the uncinate fasciculus (Kitis et al., 2012; Aristotle N Voineskos et al., 2013), inferior longitudinal fasciculus, and arcuate fasciculus (Aristotle N Voineskos et al., 2013), which is supported by network-level properties (cortical thickness correlations) of regions connected by these tracts (Wheeler et al., in press) as well as whole-brain voxel-wise investigations of white matter alterations in deficit syndrome schizophrenia (Lei et al., 2015; Spalletta et al., 2015). Early studies assessing white matter microstructure-cognition relationships focused on a limited number of tracts, such as the cingulum bundle (Kubicki et al., 2003; Nestor et al., 2013; Takei et al., 2009) or the uncinate fasciculus (Kubicki et al., 2002; Nestor et al., 2013). More recently, neurocognitive deficits have been associated with subtle, widespread tract impairment in schizophrenia (Lim et al., 2006; Spoletini et al., 2009; Aristotle N. Voineskos et al., 2013). Despite the large number of DTI studies examining negative symptoms and neurocognitive performance, a direct link between white matter tract microstructure and functional outcome in schizophrenia patients is less well-established, particularly over a period of time.
Using FA as a measure of white matter tract microstructure (Jones, 2008), our objective was to identify the neural circuitry that is related to baseline and longitudinal functional outcomes (using the Quality of Life Scale (QLS) (Heinrichs et al., 1984)) in people with schizophrenia. Our main hypotheses were: At baseline and 6-month follow-up, 1. FA of the uncinate fasciculus, arcuate fasciculus, and inferior longitudinal fasciculus in people with schizophrenia would be significantly correlated with functional outcome as indexed by total QLS score; 2. FA of these same tracts would be inversely correlated with negative symptom burden, measured using the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983); and 3. Negative symptom burden would mediate the relationship between FA of these tracts and functional outcome in schizophrenia.
2. Methods
2.1 Participants
Participants were recruited and underwent clinical assessments at the Centre for Addiction and Mental Health (CAMH) in Toronto, Canada. After receiving a complete description of the study, approved by the CAMH ethics review board, participants provided written, informed consent. Participants with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder, following administration of the Structured Clinical Interview for DSM-IV-TR Axis I Disorders and diagnostic confirmation by a trained psychiatrist (GF or ANV), comprised the schizophrenia sample. Schizophrenia (n=30) and healthy control (n=30) participants were individually matched on sex and handedness (categorized as left or right-handed based on the Edinburgh handedness inventory), and group-matched on age and parental level of education. Exclusion criteria for all subjects in this study included current substance use (verified by urine toxicology screen), history of substance dependence, head trauma with loss of consciousness, and neurological disorders. Healthy control subjects were also excluded if there was a history of primary psychotic disorder in a first-degree relative.
The Positive and Negative Symptom Scale (PANSS) (Kay et al., 1987) was administered to further characterize illness symptoms in the schizophrenia group. Negative symptoms of schizophrenia participants were assessed using the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983), cognitive function using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998), and functional outcome using the Quality of Life Scale (QLS) (Heinrichs et al., 1984). The SANS score was calculated excluding the Attention subscale from the total and inappropriate affect from the Affective Flattening subscale, as these symptoms are more closely associated with the disorganized symptom domain in schizophrenia (George Foussias et al., 2011). Similarly, the QLS total score excluded the Intrapsychic Foundations subscale, to eliminate overlap in item content of this subscale with measures of negative symptoms (Foussias et al., 2009; G Foussias et al., 2011). A number of other measures were administered to participants to further characterize the sample and to account for secondary negative symptoms. N=24 individuals also returned to repeat a number of assessments at 6-month follow-up, including the SANS and QLS (see Supplementary Methods and Table 1).
2.2 Image Acquisition
Participants underwent magnetic resonance (MR) and diffusion tensor (DT) imaging at Toronto General Hospital. DT images were acquired with a single-shot spin echo planar sequence with diffusion gradients applied in 23 noncollinear directions (b=1000s/mm2). DT images, including two baseline (b=0) images, were obtained with the following scan parameters: echo time=85.5 milliseconds, repetition time=15 000 milliseconds, field of view=330 mm, acquisition matrix=128 mm × 128 mm. Fifty-seven axial slices were acquired, with a 2.6 mm slice thickness and isotropic voxels. The entire sequence was repeated 3 times to improve the signal to noise ratio. MR images were acquired using an 8-channel head coil on a 1.5-Tesla GE Echospeed System (General Electric Medical Systems) with the following acquisition parameters: echo time=5.3 ms, repetition time=12.3 ms, time to inversion=300 ms, flip angle=20°, number of excitations=124 contiguous images with 1.5 mm thickness.
2.3 Image Processing
For DTI analysis, the 3 repetitions were coregistered to the first b=0 image in the first repetition, using the FSL FMRIB Linear Image Registration Tool to concatenate the motion corrected images. Gradients were reoriented using a weighted least squares approach. Registration corrected eddy current distortions and subject motion while averaging the three repetitions to improve the signal to noise ratio. A brain mask was then generated and deterministic whole brain tractography (Runge-Kutta second order tractography with a fixed step size of 0.5 mm) was performed at seed points in each voxel of the brain. Threshold parameters for tractography were based on the linear anisotropy measure CL: Tseed=0.3 mm, Tstop=0.15 mm, Tlength=20 mm (Westin et al., 2002). Tractography, creation of white matter fiber tracts, and clustering segmentation were performed using 3D Slicer (version 2) and Matlab (version 7.0) as previously described (Voineskos et al., 2009). Clusters were then identified to comprise fiber tracts shown to be reliably segmented with this method (Voineskos et al., 2009): bilateral uncinate fasciculus, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, arcuate fasciculus, cingulum bundle, and the genu and splenium of the corpus callosum. For each white matter tract, mean measures of fractional anisotropy were calculated using Matlab. Mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) values were also calculated for each tract and reported in Supplementary Table 2; however, the number of gradient directions acquired in this study (23), while sufficient to measure fractional anisotropy, is less than the number required for robust measures of mean diffusivity, axial diffusivity, or radial diffusivity (Jones, 2004). The T1-weighted scans were submitted to the CIVET pipeline for cortical thickness analysis followed by structural correlation analysis (Supplementary Methods).
2.4 Statistical Analysis
Supplementary Figure 1 presents a clear flowchart of imaging and statistical analyses performed on our data.
2.4.1 Principal component analysis of 12 white matter tracts
In the baseline and follow-up schizophrenia samples, dimension reduction of FA values from the 12 frontotemporal and interhemispheric white matter tracts was performed using a principal component analysis (PCA) with varimax rotation in SPSS (version 20.0.0, SPSS Inc.). The threshold for retaining components within each principal component was λ>1. This PCA was performed in preparation for examination of the relationship between white matter tract FA and QLS total scores, since there is well-established correlation of FA values among white matter tracts within each individual, and such dimension reduction reduces the number of comparisons in our main analyses.
2.4.2 Multiple Linear Regression Models
Using R (version 3.0.2), a regression model was investigated with principal component scores as independent variables; age, parental level of education, chlorpromazine equivalent dose, and duration of illness as covariates; and QLS total score as the outcome variable to test our primary hypothesis. Chlorpromazine equivalent dose and duration of illness were included as covariates to evaluate the effect of medication and chronic illness on brain structure. Regression models were similarly built with the SANS total score and RBANS total score as outcome variables to test whether to proceed with mediation. Principal component scores calculated using data from the individuals with 6-month follow-up data (n=24) were entered into similar regression models with follow-up QLS total score or SANS total score as the outcome variable. Age was not included as a covariate for the RBANS model, as the total score used was age-normed.
Where relationships were found with either the baseline SANS or RBANS total scores, exploratory analyses were then conducted with subscores. These analyses were not corrected for multiple comparisons.
2.4.3 Mediation Analysis
Mediation models were built, initially with baseline and subsequently with 6-month follow-up data, using the following specifications: independent variable = the second principal component; outcome variable = QLS total score; mediator = SANS total score; covariates = age, parental level of education, chlorpromazine equivalent dose, and duration of illness. The model was tested in the R program as outlined by Baron and Kenny's protocol (Baron and Kenny, 1986). The effect size (ab) of the mediator was calculated from the product of the partial correlations of each coefficient (a and b). The Sobel test was conducted to test for significance of this effect. To further substantiate the model, reverse causal effects were assessed by testing a feedback model (switching the outcome and mediator variables). Moderation was also assessed by including an interaction term of the independent and mediator variables in the model and re-running the analysis.
3. Results
3.1 Brain structure and circuitry differences in schizophrenia
Schizophrenia participants showed modest reductions in white matter tract FA compared to healthy controls in the left uncinate fasciculus, left arcuate fasciculus, bilateral cingulum bundle, and genu of the corpus callosum. Similarly, schizophrenia participants were characterized by reductions in cortical thickness in a number of frontal and temporal regions (Supplementary Methods and Figure 2).
3.2 Data Reduction and Relationship of FA with Functional Outcome
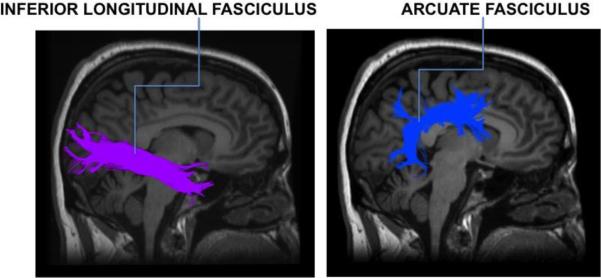
The PCA generated two components with λ>1 in both the baseline (n=30) and follow-up (n=24) samples (in both samples 61% and 11% of the variance was explained by the first and second principal component, respectively). FA of a number of white matter tracts loaded on the first component (Supplementary Materials Table 1A and 1B), except for that of the inferior longitudinal and arcuate fasciculus (Figure 1), which loaded prominently on the second component (data reported for baseline and follow-up samples, respectively: left inferior longitudinal fasciculus loading=0.81 and 0.78, right inferior longitudinal fasciculus loading=0.85 and 0.82, left arcuate fasciculus loading=0.79 and 0.85, and right arcuate fasciculus loading=0.82 and 0.84, Supplementary Table 1). The first principal component was not significantly correlated with the QLS total (baseline β=−0.25, p=0.3; follow-up β=−0.15, p=0.6) or SANS total score (baseline β=1.78, p=0.62; follow-up β=5.64, p=0.2, uncorrected). The second principal component was significantly correlated with the QLS total (baseline β=0.54, p=0.03; follow-up β=0.74, p=0.047) and SANS total scores (baseline β=−8.38, p=0.03; follow-up β=−11.3, p=0.01, uncorrected). There was no significant relationship between either principal component and the RBANS total score. Within the baseline sample, exploratory analyses showed that the second principal component was related to the SANS Avolition/Apathy (β=−2.05, p=0.05, uncorrected), and SANS Anhedonia/Asociality subscale scores (β=−2.61, p=0.04, uncorrected) in the baseline sample (Table 2).
Figure 1. Right inferior longitudinal fasciculus and arcuate fasciculus.
Sagittal view of the right inferior longitudinal fasciculus and right arcuate fasciculus as modeled by the tractography pipeline used in this study.
Table 2.
Multiple Regression Models with White Matter Tract FA
| Outcome | PC 1 β | p | PC 2 β | p | R2 |
|---|---|---|---|---|---|
| QLS Total excl. IF | −0.2539 | 0.2952 | 0.5366 | 0.0336b | 0.144 |
| SANS Total excl. Att. | 1.775 | 0.6229 | −8.3775 | 0.0278c | 0.148 |
| RBANS Totala | −2.3459 | 0.4832 | −0.2046 | 0.9481 | 0.076 |
| SANS Affective Flattening | −0.1798 | 0.9165 | −2.613 | 0.1379 | −0.013 |
| SANS Alogia | 0.7868 | 0.3456 | −1.2752 | 0.1329 | 0.012 |
| SANS Avolition/Apathy | 0.1135 | 0.9092 | −2.0455 | 0.0493c | 0.148 |
| SANS Anhedonia/Asociality | 1.0317 | 0.3953 | −2.6068 | 0.0393c | 0.078 |
PC=principal component of 12 white matter tract mean FA values, QLS Total excl. IF=Quality of Life Scale total score excluding Intrapsychic Foundations subscale, SANS Total excl. Att.=Scale for the Assessment of Negative Symptoms total score excluding Attention subscale, RBANS Total=Repeatable Battery for the Assessment of Neuropsychological Status Covariates: Age, Parental Level of Education, Chlorpormazine Equivalent Dose, Duration of Illness
Covariates: Parental Level of Education, Chlorpormazine Equivalent Dose, Duration of Illness
p < 0.05
p < 0.05, uncorrected
3.3 Negative symptoms partially mediate the relationship between tract FA and longitudinal functional outcomes
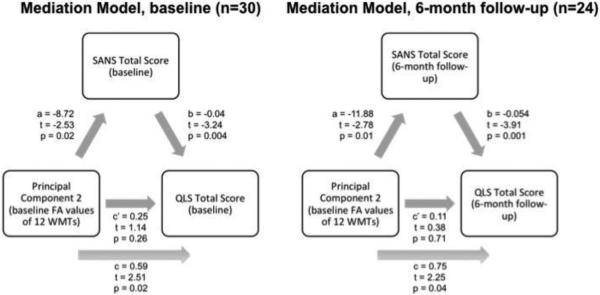
As c’<c, partial mediation of the relationship between baseline tract FA and baseline QLS total score was observed (c, the total effect=0.59; c’, the direct effect=0.25). Similarly, partial mediation of the relationship between baseline tract FA and 6-month follow-up QLS total score in the follow-up sample was observed (c=0.75, c’=0.11). The SANS total score had an effect on the relationship between the second principal component and the QLS total score (effect size ab=0.3). This effect was found to be significant by the Sobel test (z=2.0, p=0.046). No reverse causal effects were seen in the feedback model (b=−8.25, c’=−3.89). The SANS total score was not found to be a moderator of the relationship between the second principal component and the QLS total score (second principal component and SANS total score interaction term β=−0.007, p=0.65) (Figure 2).
Figure 2. Mediation Model.
Model showing the relationship between the second principal component and functional outcome in schizophrenia participants, which was partially mediated by negative symptoms. The direct effect (c’=0.25, p=0.26) was less than the total effect (c=0.59, p=0.02). Mediation was validated by the Sobel test, demonstrating a significant effect (ab=0.3, p=0.05) of negative symptoms on the relationship between neural circuitry and functional outcome. Similarly, partial mediation of the relationship between baseline tract FA and functional outcome in the follow-up sample was also observed (c=0.75, p=0.04; c’=0.11, p=0.71).
Post-hoc analyses of structural covariance between cortical regions connected by the inferior longitudinal and arcuate fasciculus revealed that patients with poorer functional outcomes demonstrated significantly altered coupling between the right inferior frontal and supramarginal gyri (Supplementary Results and Figure 3).
4. Discussion
In this study, we found that that inferior longitudinal fasciculus and arcuate fasciculus FA (represented together as a principal component) were associated with functional outcome at both baseline and 6-month follow-up. This relationship was mediated by negative symptom burden. In the context of recent findings of inferior longitudinal and arcuate fasciculus impairment in deficit syndrome patients, who exhibit severe functional impairment, our present findings extend our previous work to a clinically heterogeneous group of schizophrenia participants that may be more representative of neuroimaging schizophrenia studies. Supporting this contention, comparisons between schizophrenia participants and healthy controls showed differences in brain structure and neural circuitry reflecting those previously reported (Crespo-Facorro et al., 2000; Kubicki et al., 2007; Onitsuka T et al., 2003; Shenton et al., 2001; Wheeler and Voineskos, 2014). Therefore, on a preliminary basis, we can conclude that impairment in the inferior longitudinal and arcuate fasciculus may serve as a biomarker of functional outcome in people with schizophrenia that is stable over time.
Our study is one of the first to directly examine the relationship between white matter tract microstructure and functional outcome in a sample of schizophrenia participants. Supporting evidence for our findings come from an earlier study in prodromal patients (Karlsgodt et al., 2009), which reported decreased FA in the left hippocampus and bilateral inferior longitudinal fasciculus as predictors of poor role functioning in individuals at ultra-high risk for psychosis, while decreased FA in the right inferior longitudinal fasciculus also predicted poor social functioning in ultra-high risk subjects. This was an important finding as it demonstrated the association between the functional decline seen in antipsychotic naïve prodromal patients and FA of the inferior longitudinal fasciculus early in the course of illness. More recently, Kumar et al (Kumar et al., 2014) reported that white matter tract microstructure of the right inferior fronto-occipital fasciculus, corpus callosum, corona radiata, left cingulate gyrus, and left posterior thalamic radiation predicted social and occupational performance in patients with psychosis. It is likely that these results differ from our findings due to the inclusion of both bipolar disorder patients (who typically do not have negative symptom burden) and schizophrenia patients.
To date, the link between white matter tract impairment and poor functional outcome in schizophrenia has primarily been supported indirectly through findings in deficit syndrome patients (Kitis et al., 2012; Rowland et al., 2009; Aristotle N Voineskos et al., 2013), who are known to have enduring primary negative symptoms and particularly poor long-term outcomes (Strauss et al., 2010). Neural circuitry of deficit syndrome patients has also been explored using network analysis of cortical properties in schizophrenia (Wheeler et al., in press). Wheeler et al (Wheeler et al., in press) reported stronger frontoparietal and frontotemporal coupling in deficit syndrome patients compared to non-deficit syndrome patients and healthy control subjects. Our study results are consistent with this recent work, since in our post-hoc analysis, stronger frontoparietal coupling was found in participants with poorer functional outcome.
Analysis of our mediation model demonstrated that the relationship between white matter tract FA and functional outcome is mediated by negative symptoms. In schizophrenia, negative symptoms have been previously correlated with white matter tract impairment in frontal, temporal, and parietal regions (Asami et al., 2014; Kitis et al., 2012; Lee et al., 2013; Mitelman et al., 2009; Nakamura et al., 2012; Rowland et al., 2009; Sigmundsson et al., 2001; Szeszko et al., 2008; Aristotle N Voineskos et al., 2013; Wolkin et al., 2003). It is likely that differences in methodologies and sample populations resulted in different patterns of results in these studies. Our statistical analysis features a dimension reduction method in order to explore the contribution of all white matter tracts, since there is well-established correlation of FA values among white matter tracts within each individual. We also used our group's validated tractography pipeline (Voineskos et al., 2009), whereas other studies have used voxel-based DTI, focused on a single tract of interest, or used tract-based spatial statistics and correlation analyses in their experimental design. Our study also presents data on an outpatient sample, while some studies include both inpatients and outpatients (Nakamura et al., 2012) or solely inpatients (Szeszko et al., 2008), which could account for differences in results.
There was no relationship between either principal component and neurocognitive impairment, as measured by the RBANS total score, in this schizophrenia sample. Our group has previously built on existing evidence for the relationship between neurocognition and neural circuitry by demonstrating that widespread impairment of white matter tracts is associated with cognitive performance in schizophrenia (Aristotle N. Voineskos et al., 2013). Structural and functional studies show similar results, where cognitive performance is associated with a larger compensatory network of cortical (particularly frontal and temporal) brain regions in schizophrenia participants (Ehrlich et al., 2012; Tan et al., 2006). It is possible that in the current study we were underpowered to detect these relationships or that the use of a composite score to represent neurocognitive impairment resulted in a loss of information that may be necessary to identify the complex associations between neural circuitry and neurocognition.
Limitations of this study include the effects of medication on white matter tracts in the schizophrenia sample, as cumulative effects of medication is shown to cause reductions in white matter volumes (Ho et al., 2011) and decreased white matter FA in the parietal and occipital lobe (Szeszko et al., 2014). In an effort to account for this confounding variable, we included chlorpromazine equivalent dose as a covariate in our statistical analysis. Antipsychotic medication and duration of illness concerns are further mitigated given a previous similar finding in a prodromal sample (Karlsgodt et al., 2009). Furthermore, a future study with a larger sample size may enhance confidence in our findings and provide further clarification regarding the absence of a relationship with neurocognitive deficits in the present study.
In summary, we found that impairment in the inferior longitudinal and arcuate fasciculus, both of which were identified as being implicated in deficit syndrome schizophrenia, are associated with functional outcomes across a more heterogeneous group of schizophrenia participants. The results of our study provide preliminary evidence for brain-based biomarkers of functional outcome in people with schizophrenia. If confirmed, our findings may assist in neuroimaging-guided early detection efforts and in determining long-term prognosis. Targeting the identified brain circuitry in treatment studies may improve negative symptoms and functional outcome in people with schizophrenia.
Supplementary Material
Table 1.
Sociodemographic and clinical characteristics of 30 schizophrenia and 30 HC subjects
| Schizophrenia Mean (SD) | HC Mean (SD) | t | p | |
|---|---|---|---|---|
| Age (years) | 39.3 (12.9) | 39.3 (12.6) | 0.0101 | 0.9919 |
| Sex (M:F) | 18:12 | 18:12 | - | - |
| Ethnicity | ||||
| Caucasian (n) | 19 | 28 | - | - |
| Asian (n) | 5 | 1 | - | - |
| African American (n) | 4 | 0 | - | - |
| Other (n) | 2 | 1 | - | - |
| Education (years) | 13.8 (2.2) | 15.1 (2.0) | 2.42 | 0.01875a |
| Parental Level of Education | 4.1 (2.3) | 4.9 (2.0) | 1.4302 | 0.1581 |
| Handedness (L:R) | 1:29 | 1:29 | - | - |
| IQ (WTAR) | 108.2 (16.8) | 115.1 (9.0) | 1.9648 | 0.05595 |
| MMSE | 28.9 (1.7) | 29.5 (0.7) | 1.65 | 0.1062 |
| Duration of Illness (years) | 15.4 (13.4) | - | - | - |
| CIRS-G | 1.2 (0.6) | 0.7 (0.7) | 2.6824 | 0.009665 |
| Diagnosis (schizoaffective:schizophrenia) | 8:22 | - | - | - |
| Chlorpromazine Equivalents (mg/day)b | 189.5 (193.5) | - | - | - |
| CDSS | 2.5 (3.3) | - | - | - |
| AIMS | 0.2 (0.6) | - | - | - |
| SAS | 1.3 (2.0) | - | - | - |
| BAS | 0.7 (1.8) | - | - | - |
| Systolic BP | 121.2 (14.9) | 122.1 (12.3) | 0.2317 | 0.8178 |
| Diastolic BP | 74.4 (15.6) | 75.21 (10.5) | 0.2316 | 0.8179 |
| Weight (kg) | 81.5 (18.5) | 74.4 (15.8) | 1.5782 | 0.1202 |
| Height (m) | 1.7 (0.1) | 1.7 (0.1) | 0.2797 | 0.7808 |
| SANS Total excl. Attention (baseline, n=30) | 25.9 (18.2) | - | - | - |
| SANS Total excl. Attention (6-month follow-up, n=24) | 23.6 (19.8) | - | - | - |
| QLS Total excl. Intrapsychic Foundations (baseline, n=30) | 3.4 (1.2) | - | - | - |
| QLS Total excl. Intrapsychic Foundations (6-month follow-up, n=24) | 3.3 (1.4) | - | - | - |
| RBANS Total | 82.5 (15.6) | 96.6 (8.4) | 4.2106 | 0.0001356a |
| PANSS | ||||
| Positive | 11.1 (4.4) | - | - | - |
| Negative | 12.4 (5.6) | - | - | - |
| General | 21.9 (6.2) | - | - | - |
HC=healthy controls; WTAR=Wechsler Test for Adult Reading; MMSE=Mini-Mental State Examination; CIRS-G=Cumulative Illness Rating Scale for Geriatrics; CDSS=Calgary Depression Scale for Schizophrenia; AIMS=Abnormal Involuntary Movement Scale; SAS=Symptom Angus Scale; BAS=Barnes Akathisia Scale
p < 0.05
n=8 individuals were not taking antipsychotic medication at the time of assessment
Acknowledgements
This work was supported by the CAMH Foundation (thanks to the Kimel Family, the Koerner New Scientist Award, and the Paul E. Garfinkel New Investigator Catalyst Award); the Canadian Institutes of Health Research; Ontario Mental Health Foundation; Brain and Behaviour Research Foundation; and National Institute of Mental Health (R01MH099167).
Role of the Funding Source:
None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Tina Behdinan wrote the manuscript, contributed to image processing and conducted the statistical analysis
George Foussias recruited participants and conducted clinical and neuropsychological assessments
Anne Wheeler contributed to image processing and performing the cortical thickness statistical analysis
Laura Stefanik recruited participants and conducted clinical and neuropsychological assessments
Daniel Felsky contributed to image processing
Gary Remington supervised participant recruitment and collection of clinical and neuropsychological data
Tarek Rajji supervised participant recruitment and collection of clinical and neuropsychological data
Mallar Chakravarty supervised image processing
Aristotle Voineskos is the principal investigator, and contributed to manuscript writing, image processing, and statistical analysis
Conflict of Interest Disclosures: Dr. Foussias has served on advisory boards for Roche, and has received speaker fees from Roche, Novartis, and Lundbeck. Dr. Remington has received consultancy fees from Neurocrine, Synchroneuron, and Novartis, as well as research and speaker fees from Novartis.
References
- Andreasen N. Scale for the Assessment of Negative Symptoms (SANS) 1983 [PubMed] [Google Scholar]
- Asami T, Hyuk Lee S, Bouix S, Rathi Y, Whitford TJ, Niznikiewicz M, Nestor P, McCarley RW, Shenton ME, Kubicki M. Cerebral white matter abnormalities and their associations with negative but not positive symptoms of schizophrenia. Psychiatry Res. Neuroimaging. 2014;222:52–59. doi: 10.1016/j.pscychresns.2014.02.007. doi:10.1016/j.pscychresns.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. doi:10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Horan WP, Collins LM. Examining the latent structure of negative symptoms: is there a distinct subtype of negative symptom schizophrenia? Schizophr. Res. 2005;77:151–165. doi: 10.1016/j.schres.2005.03.022. doi:10.1016/j.schres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Breier A, Schreiber JL, Dyer J, Pickar D. National institute of mental health longitudinal study of chronic schizophrenia: Prognosis and predictors of outcome. Arch. Gen. Psychiatry. 1991;48:239–246. doi: 10.1001/archpsyc.1991.01810270051007. doi:10.1001/archpsyc.1991.01810270051007. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am. J. Psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, O'Leary DS, Bockholt HJ, Magnotta V. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr. Res. 2000;46:35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Brauns S, Yendiki A, Ho B-C, Calhoun V, Schulz SC, Gollub RL, Sponheim SR. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr. Bull. 2012;38:1050–1062. doi: 10.1093/schbul/sbr018. doi:10.1093/schbul/sbr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The Neurodevelopmental Hypothesis of Schizophrenia, Revisited. Schizophr. Bull. 2009:sbn187. doi: 10.1093/schbul/sbn187. doi:10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussias G, Agid O, Remington G. Negative Symptoms Across the Schizophrenia Spectrum: Phenomenological and Neurobiological Perspectives. In: Ritsner M, editor. Handbook of Schizophrenia Spectrum Disorders, Volume II. Springer; Netherlands: 2011. pp. 1–32. [Google Scholar]
- Foussias G, Mann S, Zakzanis KK, van Reekum R, Agid O, Remington G. Prediction of longitudinal functional outcomes in schizophrenia: the impact of baseline motivational deficits. Schizophr. Res. 2011;132:24–27. doi: 10.1016/j.schres.2011.06.026. doi:10.1016/j.schres.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Foussias G, Mann S, Zakzanis KK, van Reekum R, Remington G. Motivational deficits as the central link to functioning in schizophrenia: a pilot study. Schizophr. Res. 2009;115:333–337. doi: 10.1016/j.schres.2009.09.020. doi:10.1016/j.schres.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr. Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr. Bull. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. doi:10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two-year outcome in first-episode schizophrenia: predictive value of symptoms for quality of life. Am. J. Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex J. Devoted Study Nerv. Syst. Behav. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. doi:10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn. Reson. Med. 2004;51:807–815. doi: 10.1002/mrm.20033. doi:10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White Matter Integrity and Prediction of Social and Role Functioning in Subjects at Ultra-High Risk for Psychosis. Biol. Psychiatry, Cortical Development and Glutamatergic Dysregulation in Schizophrenia. 2009;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. doi:10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Flszbein A, Opfer LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. doi:10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Kitis O, Ozalay O, Zengin EB, Haznedaroglu D, Eker MC, Yalvac D, Oguz K, Coburn K, Gonul AS. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry Clin. Neurosci. 2012;66:34–43. doi: 10.1111/j.1440-1819.2011.02293.x. doi:10.1111/j.1440-1819.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin C-F, Park H-J, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. doi:10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin C-F, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate Fasciculus Findings in Schizophrenia: A Magnetic Resonance Diffusion Tensor Imaging Study. Am. J. Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. doi:10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin C-F, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol. Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. doi:10.1016/S0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Iwabuchi S, Oowise S, Balain V, Palaniyappan L, Liddle PF. Shared white-matter dysconnectivity in schizophrenia and bipolar disorder with psychosis. Psychol. Med. FirstView. 2014:1–12. doi: 10.1017/S0033291714001810. doi:10.1017/S0033291714001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton ME. Extensive white matter abnormalities in patients with first-episode schizophrenia: A diffusion tensor imaging (DTI) study. Schizophr. Res. 2013;143:231–238. doi: 10.1016/j.schres.2012.11.029. doi:10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Li N, Deng W, Li M, Huang C, Ma X, Wang Q, Guo W, Li Y, Jiang L, Zhou Y, Hu X, Mary McAlonan G, Li T. White matter alterations in first episode treatment-naïve patients with deficit schizophrenia: a combined VBM and DTI study. Sci. Rep. 2015;5:12994. doi: 10.1038/srep12994. doi:10.1038/srep12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MD, Kelvin, Ardekani PD, Babak, Nierenberg MD, Ph.D., Jay, Butler PD, Pamela, Javitt MD, Ph.D., Daniel, Hoptman PD, Matthew Voxelwise Correlational Analyses of White Matter Integrity in Multiple Cognitive Domains in Schizophrenia. Am. J. Psychiatry. 2006;163:2008–2010. doi: 10.1176/appi.ajp.163.11.2008. doi:10.1176/appi.ajp.163.11.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Ho B-C, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. doi:10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Canfield EL, Newmark RE, Brickman AM, Torosjan Y, Chu K-W, Hazlett EA, Haznedar MM, Shihabuddin L, Buchsbaum MS. Longitudinal Assessment of Gray and White Matter in Chronic Schizophrenia: A Combined Diffusion-Tensor and Structural Magnetic Resonance Imaging Study. Open Neuroimaging J. 2009;3:31–47. doi: 10.2174/1874440000903010031. doi:10.2174/1874440000903010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawasaki Y, Takahashi T, Furuichi A, Noguchi K, Seto H, Suzuki M. Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: a voxel-based diffusion tensor imaging study. Psychiatry Res. 2012;202:233–238. doi: 10.1016/j.pscychresns.2011.09.006. doi:10.1016/j.pscychresns.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Nakamura M, Niznikiewicz M, Levitt JJ, Shenton ME, McCarley RW. Neuropsychological variability, symptoms, and brain imaging in chronic schizophrenia. Brain Imaging Behav. 2013;7:68–76. doi: 10.1007/s11682-012-9193-0. doi:10.1007/s11682-012-9193-0. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Kasai K, et al. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Arch. Gen. Psychiatry. 2003;60:349–355. doi: 10.1001/archpsyc.60.4.349. doi:10.1001/archpsyc.60.4.349. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. doi:10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Leslie D, Keefe R, McEvoy J, Swartz M, Perkins D, Stroup S, Hsiao JK, Lieberman J, CATIE Study Investigators Group Barriers to employment for people with schizophrenia. Am. J. Psychiatry. 2006;163:411–417. doi: 10.1176/appi.ajp.163.3.411. doi:10.1176/appi.ajp.163.3.411. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White matter alterations in deficit schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009;34:1514–1522. doi: 10.1038/npp.2008.207. doi:10.1038/npp.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am. J. Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Spalletta G, De Rossi P, Piras F, Iorio M, Dacquino C, Scanu F, Girardi P, Caltagirone C, Kirkpatrick B, Chiapponi C. Brain white matter microstructure in deficit and non-deficit subtypes of schizophrenia. Psychiatry Res. Neuroimaging. 2015;231:252–261. doi: 10.1016/j.pscychresns.2014.12.006. doi:10.1016/j.pscychresns.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Spoletini I, Cherubini A, Di Paola M, Banfi G, Rüsch N, Martinotti G, Bria P, Rubino IA, Siracusano A, Caltagirone C, Spalletta G. Reduced fronto-temporal connectivity is associated with frontal gray matter density reduction and neuropsychological deficit in schizophrenia. Schizophr. Res. 2009;108:57–68. doi: 10.1016/j.schres.2008.11.011. doi:10.1016/j.schres.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophr. Bull. 2010;36:788–799. doi: 10.1093/schbul/sbn167. doi:10.1093/schbul/sbn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz-Bruce H, Kane JM, Bilder RM. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. doi:10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, Malhotra AK. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:1324–1331. doi: 10.1038/npp.2013.288. doi:10.1038/npp.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr. Res. 2009;114:119–127. doi: 10.1016/j.schres.2009.05.012. doi:10.1016/j.schres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Tan H-Y, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, Weinberger DR, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am. J. Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. doi:10.1176/appi.ajp.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: A meta-analysis. Schizophr. Res. 2009;113:189–199. doi: 10.1016/j.schres.2009.03.035. doi:10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Felsky D, Kovacevic N, Tiwari AK, Zai C, Chakravarty MM, Lobaugh NJ, Shenton ME, Rajji TK, Miranda D, Pollock BG, Mulsant BH, McIntosh AR, Kennedy JL. Oligodendrocyte Genes, White Matter Tract Integrity, and Cognition in Schizophrenia. Cereb. Cortex. 2013;23:2044–2057. doi: 10.1093/cercor/bhs188. doi:10.1093/cercor/bhs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70:472–480. doi: 10.1001/jamapsychiatry.2013.786. doi:10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, O'Donnell LJ, Lobaugh NJ, Markant D, Ameis SH, Niethammer M, Mulsant BH, Pollock BG, Kennedy JL, Westin CF, Shenton ME. Quantitative examination of a novel clustering method using magnetic resonance diffusion tensor tractography. NeuroImage. 2009;45:370–376. doi: 10.1016/j.neuroimage.2008.12.028. doi:10.1016/j.neuroimage.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin C-F, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med. Image Anal. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front. Hum. Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. doi:10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Wessa M, Szeszko PR, Foussias G, Chakravarty MM, Lerch JP, DeRosse P, Remington G, Mulsant B, Linke J, Malhotra AK, Voineskos AN. Brain network investigation in schizophrenia, bipolar disorder and healthy controls reveals prominent network differences in deficit-subtype schizophrenia. JAMA Psychiatry. in press. [Google Scholar]
- Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am. J. Psychiatry. 2003;160:572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.