Abstract
Purpose
The purposes of the present study are to assess the clinical efficiency of Piezo-intracytoplasmic sperm injection (ICSI) and to improve the Piezo-ICSI method for human oocytes.
Methods
We examined three ICSI methods to determine their clinical efficiency by comparing the survival, fertilization, good-quality day-3 embryo, pregnancy, and live birth rates. The three ICSI methods tested were conventional ICSI (CI) (using beveled spiked micropipettes with a wall thickness of 1 μm), conventional Piezo-ICSI (CPI) (using flat-tipped micropipettes with a wall thickness of 0.925 μm), and improved Piezo-ICSI (IPI) (using flat-tipped micropipettes with a wall thickness of 0.625 μm). We collectively investigated 2020 mature oocytes retrieved from 437 patients between October 2010 and January 2014.
Results
The survival rates after CI, CPI, and IPI were 90, 95, and 99 %, respectively. The fertilization rates after CI, CPI, and IPI were 68, 75, and 89 %, respectively. The good-quality day-3 embryo rates after CI, CPI, and IPI were 37, 43, and 55 %, respectively. The pregnancy rates after the transfer of good-quality day-3 embryo of CI, CPI, and IPI were 19, 21, and 31 %, respectively. The live birth rates of CI, CPI, and IPI were 15, 16, and 25 %, respectively. Significantly higher survival, fertilization, good-quality day-3 embryo, pregnancy, and live birth rates were obtained using IPI.
Conclusions
When comparing the IPI to the CI and CPI, the results revealed that the Piezo-ICSI using flat-tipped micropipettes with a wall thickness of 0.625 μm significantly improves survival, fertilization, good-quality day-3 embryo, pregnancy, and live birth rates.
Keywords: Fertilization, Human, ICSI, Piezo, Lysis, Wall thickness
Introduction
The first four pregnancies achieved by intracytoplasmic sperm injection (ICSI) were reported in 1992 [1]. At that time, the clinical application using ICSI was only for male factor infertility, but today, it is the treatment of choice in cases of failed conventional IVF cycles. The ICSI has now been become an essential technique in assisted reproductive technology.
The ICSI has been widely performed by the mechanical penetration of zona pellucida and the membrane by using beveled and spiked micropipettes (Fig. 1a) where the cytoplasm is aspirated into the micropipette to break the membrane. After membrane breakage, the sperm is injected into the cytoplasm (conventional ICSI).
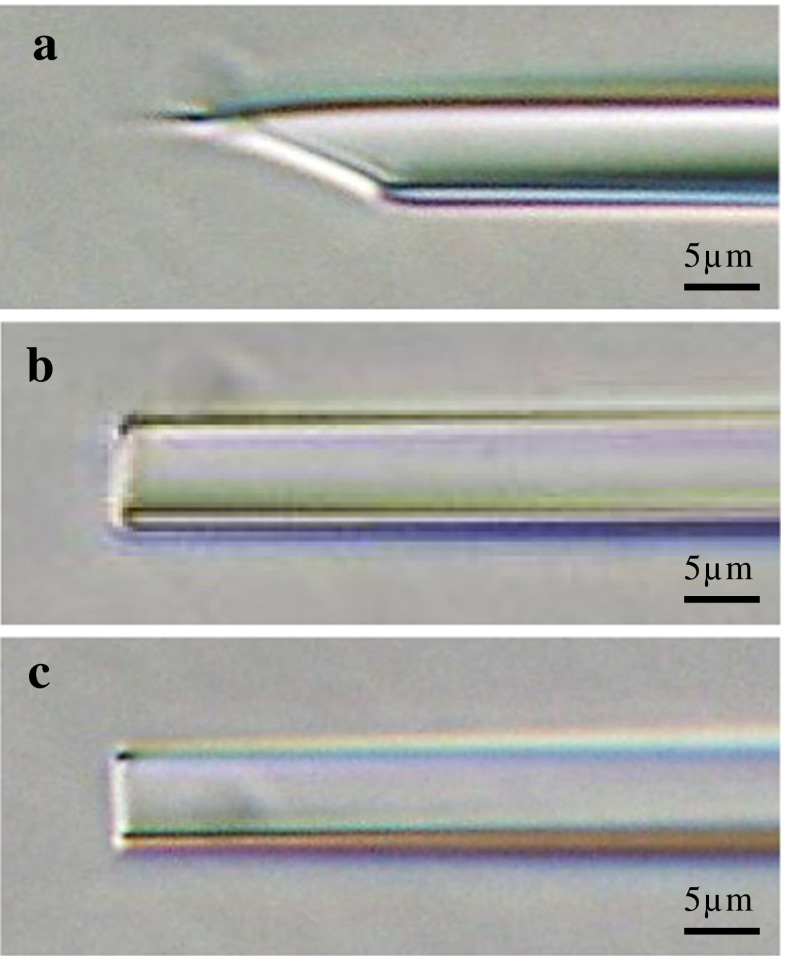
Fig. 1.
Photographs of micropipettes. a Micropipette used for conventional ICSI with a beveled and spiked tip (5-μm inner diameter and 1-μm wall thickness: micro-injection pipette, K-MPIP-1035, Cook Ireland Ltd., Ireland). b Micropipette used for conventional Piezo-ICSI with flat tip (5-μm inner diameter and 0.925-μm wall thickness: PIN07-20FT, PRIME TECH Ltd., Japan). c Micropipette used for improved Piezo-ICSI (5-μm inner diameter and 0.625-μm wall thickness: PINU06-20FT, PRIME TECH Ltd., Japan)
Kimura and Yanagimachi performed the membrane breakage by applying a Piezo pulse which produced ultra-fast submicron forward momentum using uniquely shaped flat-tipped micropipettes with no bevel or spike (Fig. 1b, c) (Piezo-ICSI) [2]. Huang et al. applied the Piezo-ICSI to human oocytes for the first time and reported that the fertilization rate of eggs injected (60.5 %), damage rate (7.6 %), abnormality rate (3 %), and ongoing pregnancy rate beyond 8 weeks (28.2 %) were comparable to those for other techniques that have been successfully reported [3]. After Yanagida et al. used the Piezo-ICSI on human oocytes, they reported significant improvement of both survival (81 vs 89 %) and fertilization (54 vs 70 %) rates when compared to the conventional ICSI [4]. Takeuchi et al. reported that the Piezo-ICSI on human oocytes demonstrated significantly more favorable results, with a fertilization rate of 90.3 %, where the conventional ICSI was 83.1 %. The cleavage rate after Piezo-ICSI was 88.1 %, where the cleavage rate after conventional ICSI was 84.6 % [5]. However, to the best of our knowledge, reports of the Piezo-ICSI applied to human oocytes are limited to these three reports and the clinical efficiency of the Piezo-ICSI on human oocytes has not been well clarified. The objectives of this study were to assess the clinical efficiency of the Piezo-ICSI and to improve the Piezo-ICSI method for human oocytes.
Materials and methods
We examined three ICSI methods to determine their clinical efficiency. We compared the volume of cytoplasm aspirated into the micropipette at membrane breakage, the size of injection site in the oocyte membrane, survival, fertilization, good-quality day-3 embryo, pregnancy, and live birth rates after ICSI. The three ICSI methods examined were conventional ICSI (CI) using beveled and spiked micropipettes with a wall thickness of 1 μm, conventional Piezo-ICSI (CPI) using flat-tipped micropipettes with a wall thickness of 0.925 μm, and improved Piezo-ICSI (IPI) using flat-tipped micropipettes with a wall thickness of 0.625 μm. We retrospectively investigated 2020 mature oocytes retrieved from 437 patients who were attending at Niji Clinic between October 2010 and January 2014. Of these, 624 mature oocytes retrieved from 120 patients were microinjected using CI, 717 mature oocytes retrieved from 166 patients were microinjected using CPI, and 679 mature oocytes retrieved from 151 patients were microinjected using IPI. Two ovarian stimulation protocols were used for patients. One was the clomiphene citrate protocol, and the other one was GnRH antagonist protocol [6, 7]. Final oocyte maturation was achieved by 10,000 IU of hCG. Oocyte retrieval was carried out by vaginal ultrasound, 35 h after hCG administration. Retrieved oocytes were cultured in Sydney IVF Fertilization Medium (K-SIFM-20, COOK, Australia). Denudation of the oocytes was conducted 3 h after oocyte retrieval. Only metaphase II oocytes at the time of the denudation were microinjected.
Conventional intracytoplasmic sperm injection
We used commercially available ICSI micropipettes with a beveled and spiked tip (Fig. 1a) (K-MPIP-1035, Cook Ireland Ltd., Ireland). The inner diameter of the micropipette was 5 μm, and the wall thickness was 1 μm. The micropipette was connected to a pneumatic injector (IM-9C, NARISHIGE Inc., Japan). The preparation for filling the micropipette was done as follows. First, we aspirated Hepes-buffered medium (SYDNEY IVF GAMETE BUFFER, Cook Australia Pty Ltd., Australia) into the micropipette by capillary action for 1 min; then, we aspirated 7 % polyvinylpyrrolidone (7 % PVP Solution, Irvine Scientific, USA) applying negative pressure using pneumatic injector. A motile sperm was immobilized by crushing the tail with the tip of the micropipette and aspirated tail first into the micropipette with in a 10-μl drop of 7 % PVP. With the polar body at 12 o’clock, the micropipette was inserted through the zona pellucida (Fig. 3a–c) far into the oocyte (~90 % of the oocyte diameter) to stretch the membrane. The membrane breakage procedure was performed as follows. Air was aspirated into the micropipette using a pneumatic injector to create negative pressure and suction onto the membrane. At first, the membrane was slowly aspirated into the micropipette until a sudden flow of cytoplasm into the micropipette occurred. We consider this to be the moment of the membrane breakage. After membrane breakage, positive air pressure was quickly provided to stop the flow of cytoplasm into the micropipette. During this procedure, the farthest point reached by the aspirated cytoplasm in the micropipette was observed. After membrane breakage, we introduced positive pressure using pneumatic injector to transfer the sperm into the oocyte.
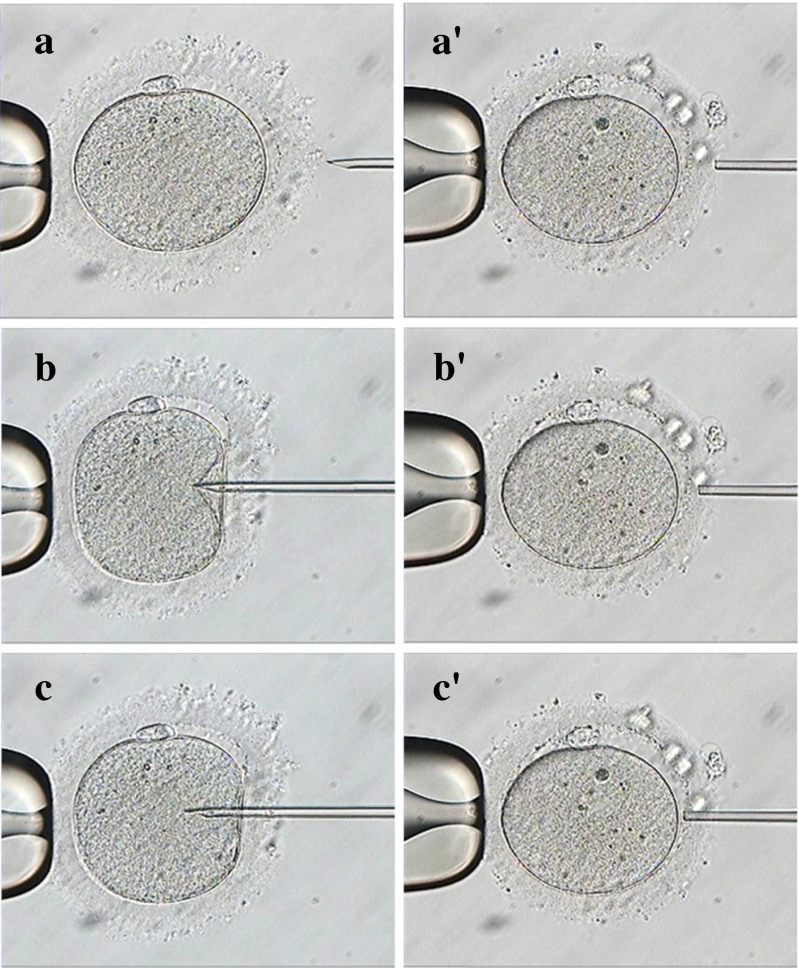
Fig. 3.
Zona drilling of conventional ICSI (CI) and Piezo-ICSI (PI) prior to ICSI. CI: before drilling (a), during drilling (b), and after drilling (c). PI: before drilling (a’), during drilling (b’), and after drilling (c’)
Conventional Piezo-intracytoplasmic sperm injection
We used commercially available Piezo-ICSI micropipettes with a flat tip (Fig. 1b) (PIN07-20FT, PRIME TECH Ltd., Japan). The inner diameter of the micropipette was 5 μm, and the wall thickness was 0.925 μm. Of Fluorinert (FC-770, 3 M), 6.25 μl was placed in the middle of the micropipette. The micropipette was inserted and clamped into the micropipette holder which was then connected to the hydraulic injector (HDJ-M3, PRIME TECH Ltd.). The Piezo-micromanipulator drive unit (Fig. 2) (MB-U, PRIME TECH Ltd.) was attached to the micropipette holder (Fig. 2) (MB-U, PRIME TECH Ltd.). The Piezo drive unit was driven by a controller (PMAS-ET150, PRIME TECH Ltd.). After Fluorinert was pushed to the tip of the micropipette, 6–12 pl of 7 % PVP was aspirated into the micropipette. The sperm was then immobilized, as was done for the conventional ICSI, and aspirated tail first into the micropipette. Without oocyte deformation, the micropipette was gently placed against the zona pellucida while Piezo pulses were applied (speed 6, intensity 2), to allow the pipette to break through the zona pellucida and not the membrane (Fig. 3a’–c’). The sperm was advanced until the sperm head was near the tip of the micropipette, and the micropipette was advanced forward (to ~90 % of the oocyte diameter) to stretch the membrane. The breakage of the membrane was performed by applying one Piezo pulse (speed 1, intensity 2) without aspirating the cytoplasm into the micropipette, and the sperm was injected into the oocyte.
Fig. 2.
Drive unit of a Piezo-micromanipulator (MB-U, PRIME TECH Ltd., Japan) attached to the micropipette holder
Improved Piezo-intracytoplasmic sperm injection
We used commercially available ultra-thin-walled Piezo-ICSI micropipettes with a flat tip (Fig. 1c) (PINU06-20FT, PRIME TECH Ltd., Japan). The inner diameter of the micropipette was 5 μm, and the wall thickness was 0.625 μm. Aside from using ultra-thin-walled Piezo-ICSI micropipettes, the same ICSI method for the CPI was used.
Embryo culture and assessment
The day of oocyte retrieval was considered as day 0. After ICSI, oocytes were cultured individually from day 1 to day 3 in 15 μl droplets of cleavage medium (Cook Australia Pty Ltd., Australia) covered by mineral oil (OVOIL, Vitrolife, Sweden). The fertilization was confirmed on day 1 (24–25 h after oocyte retrieval) by the presence of two pronuclei. All oocytes and embryos were incubated at 37 °C in an atmosphere of 6 % CO2, 5 % O2, and 89 % N2. On day 3, embryos originating from normally fertilized zygotes were observed. Good-quality embryos were defined as those having regular blastomeres, with <20 % fragments and those containing at least seven cells. On day 3, a single good-quality embryo was transferred. Fresh embryo transfer was cancelled for some patients in order to avoid potential risks arising from ovarian hyperstimulation syndrome or because of a thin (<8 mm) endometrium at the time of the oocyte retrieval, and one to three good-quality embryos were cryopreserved. The embryos were cryopreserved by a previously described method, with slight modifications, using a cryotop (Kitazato Supply Co., Fujinomiya, Japan) [8, 9]. Single cryopreserved-warmed good-quality day-3 embryo transfer was performed on day-3 endometrium in spontaneous cycle or in hormonal replace treatment cycle. When the gestational sac was recognized in the uterus using ultrasound, it was defined as a clinical pregnancy.
Comparing CI, CPI, and IPI methods
The volume of cytoplasm aspirated into the micropipette at membrane breakage of CI, CPI, and IPI and the size of injection site in the membrane of CPI and IPI were compared. The survival; fertilization; good-quality day-3 embryo; pregnancy; implantation; and live birth rates of CI, CPI, and IPI were also compared. In order to minimize the possibility of technique and other technically related factors influencing the results, all the ICSI procedures were performed by the same embryologist who was well experienced in performing the Piezo-ICSI and conventional ICSI.
Volume of cytoplasm aspirated into the micropipette
The volume of cytoplasm aspirated into the micropipette using CI was calculated as follows. Although the same embryologist exerted the same air volume of negative pressure for micropipette, the volume of cytoplasm aspirated into the micropipette varied at every oocyte because of variation of elasticity of membrane each oocyte. We measured the distance reached by the aspirated cytoplasm relative to the micropipette tip, as well as the micropipette dimensions (inner diameter at the tip of the micropipette and at the farthest point reached by the aspirated cytoplasm) on a monitor. The distance reached by the aspirated cytoplasm was converted to volume (in μl) using a micrometer scale under the microscope at the same magnification.
Using CPI and IPI methods, membrane breakage was performed by applying one Piezo pulse and zero negative pressure, which resulted in no aspiration of cytoplasm into the micropipette. Therefore, the volume of cytoplasm aspirated into the micropipette of CPI and IPI was defined as <0>.
Size of injection site in the membrane
The injection site in the membrane using CPI and IPI was calculated by subtracting the area of inner diameter of the micropipette tip from the area of outer diameter of the micropipette tip.
Statistical analysis
The one-way ANOVA and multiple comparison tests, the chi-squared test, and Fisher’s exact test were used to determine statistical differences between groups. A P value of <0.05 was considered significant.
Results
Table 1 summarizes the patient’s demographic characteristics. Among the three groups in the mean age of women at the time of ICSI, there were no significant differences when analyzing the rates of primary infertility, the cause of infertility, and the ovarian stimulation protocols.
Table 1.
Demographic characteristics of women in the conventional ICSI (CI), conventional Piezo-ICSI (CPI), and improved Piezo-ICSI (IPI)
| CI | CPI | IPI | |
|---|---|---|---|
| No. of women | 120 | 166 | 151 |
| Mean age of women at ICSI (years) ± SD | 38.8 ± 3.9 | 39.2 ± 4.4 | 39.0 ± 4.5 |
| Mean no. of prior stimulated cycles ± SD | 3.3 ± 4.3 | 3.2 ± 3.5 | 3.1 ± 3.3 |
| No. with primary infertility (%) | 85 (71) | 116 (70) | 108 (72) |
| No. with secondary infertility (%) | 35 (29) | 50 (30) | 43 (28) |
| Cause of infertility | |||
| Tuboperitoneal (%) | 13 (11) | 17 (10) | 16 (11) |
| Endometriosis (%) | 12 (10) | 21 (13) | 18 (12) |
| Male (%) | 36 (30) | 48 (29) | 42 (28) |
| Unexplained (%) | 59 (49) | 80 (48) | 75 (50) |
| Ovarian stimulation protocol | |||
| Clomiphene citrate (%) | 60 (50) | 86 (52) | 76 (50) |
| GnRH antagonist (%) | 60 (50) | 80 (48) | 75 (50) |
No significant difference was observed in demographic characteristics among the three methods (P > 0.05)
Table 2 summarizes the volume of cytoplasm aspirated into the micropipette and the size of injection site in the membrane. The volumes of cytoplasm aspirated into the micropipette at membrane breakage (mean ± SD) of CI, CPI, and IPI were 2746 ± 940, 0 ± 0, and 0 ± 0 μm3, respectively. A significantly more volume of cytoplasm aspirated into the micropipette was observed using CI as compared to the CPI and IPI (P < 0.0001). The injection sites in the membrane using CPI and IPI were 17 and 11 μm2, respectively. The injection site using IPI was 35 % smaller than when performing the CPI.
Table 2.
Inner diameter of micropipette, wall thickness of micropipette, volume of cytoplasm aspirated into the micropipette, and injection site in the membrane using conventional ICSI (CI), conventional Piezo-ICSI (CPI), and improved Piezo-ICSI (IPI)
| CI | CPI | IPI | |
|---|---|---|---|
| Inner diameter of micropipette (μm) | 5 | 5 | 5 |
| Wall thickness of micropipette (μm) | 1 | 0.925 | 0.625 |
| Mean volume of cytoplasm aspirated into the micropipette (μm3) ± SD | 2746 ± 940 a | 0 ± 0 b | 0 ± 0 b |
| Injection site in the membrane (μm2) | – | 17 | 11 |
Values with a different letter in the same column differ significantly (P < 0.05)
Table 3 summarizes the survival, fertilization, good-quality day-3 embryo, pregnancy, implantation, and live birth rates. The survival rates of CI, CPI, and IPI were 90, 95, and 99 %, respectively. A significantly higher survival rate was achieved using CPI as compared to the CI (P = 0.0004). Also, a significantly higher survival rate was obtained using IPI as compared to the CPI (P < 0.0001).
Table 3.
ICSI results, embryo development, pregnancy, and live birth rates using conventional ICSI (CI), conventional Piezo-ICSI (CPI), and improved Piezo-ICSI (IPI)
| CI | CPI | IPI | |
|---|---|---|---|
| No. of oocytes received ICSI | 624 | 717 | 679 |
| No. of oocytes survived after ICSI (%) | 559 (90) a | 680 (95) b | 675 (99) c |
| No. of oocytes fertilized (%) | 424 (68) a | 537 (75) b | 607 (89) c |
| No. of good-quality day-3 embryos (%) | 233 (37) a | 311 (43) b | 371 (55) c |
| No. of cycles of good-quality day-3 embryo transfer | 166 | 153 | 148 |
| No. of clinical pregnancies (%) | 31 (19) a | 32 (21) a | 46 (31) b |
| No. of good-quality day-3 embryos transferred | 166 | 153 | 148 |
| No. of embryos implanted (%) | 31 (19) a | 32 (21) a | 46 (31) b |
| No. of live births (%) | 25 (15) a | 24 (16) a | 37 (25) b |
Values with a different letter in the same column differ significantly (P < 0.05)
The fertilization rates per injected oocytes after CI, CPI, and IPI were 68, 75, and 89 %, respectively. A significantly higher fertilization rate was achieved using CPI as compared to the CI (P = 0.0052). Also, a significantly higher fertilization rate was obtained using IPI as compared to the CPI (P < 0.0001). The good-quality day-3 embryo rates per injected oocytes of CI, CPI, and IPI were 37, 43, and 55 %. A significantly higher good-quality day-3 embryo rate was achieved using CPI as compared to the CI (P = 0.0258). Also, a significantly higher good-quality day-3 embryo rate was obtained using IPI as compared to the CPI (P < 0.0001). The pregnancy rates after the transfer of a single good-quality fresh or cryopreserved day-3 embryo of CI, CPI, and IPI were 19, 21, and 31 %. A significantly higher pregnancy rate was achieved using IPI as compared to the CI (P = 0.0125) and CPI (P = 0.0490). The live birth rates of CI, CPI, and IPI were 15, 16, and 25 %. A significantly higher live birth rate was obtained using IPI as compared to the CI (P = 0.0330) and CPI (P = 0.0463).
Discussion
This study demonstrates that the Piezo-ICSI using micropipettes with a wall thickness of 0.925 μm significantly improve the survival, fertilization, and good-quality day-3 embryo rates when compared to the conventional ICSI. It also shows that the Piezo-ICSI using micropipettes with a wall thickness of 0.625 μm significantly improves the survival, fertilization, good-quality day-3 embryo, pregnancy, and live birth rates when compared to the conventional ICSI and Piezo-ICSI using micropipettes with a wall thickness of 0.925 μm. As the present study is retrospective, several biases may have been introduced that may cast doubt on the conclusions. However, there were no changes in the clinical and laboratory protocols during the study period. Similar technique and the same catheters were used for transferring embryos. Laboratory and clinical personnel remained the same. There was no difference among the three groups, including the mean age of women at the time of ICSI, rates of primary infertility, cause of infertility, and ovarian stimulation protocols. Therefore, we believe that it is unlikely that any significant bias was introduced.
In the present study, significantly higher survival and fertilization rates were observed when using CPI as compared to the CI (survival rates 90 vs 95 %, fertilization rates 68 vs 75 %). These results are congruent with those of Yanagida et al. [4] who tested the Piezo-ICSI for human oocytes and reported significant improvement of both survival and fertilization rates as compared to the conventional ICSI (survival rates 81 vs 89 %, fertilization rates 54 vs 70 %). In the present study, the volumes of cytoplasm aspirated into the micropipette using CI and CPI were 2746 ± 940 and 0 ± 0 μm3, respectively. When using CI method, the injection site in the membrane was larger due to the procedure of aspirating the cytoplasm into the micropipette during membrane breakage, which is avoided when using Piezo-ICSI. In addition, this procedure might also increase the physical damage to the oocyte. As a result, the survival and fertilization rates using CI were significantly lower as compared to the CPI. Furthermore, the micropipette was allowed to penetrate the zona pellucida alone without membrane deformation as the Piezo pulses were applied (Fig. 1a’–c’) when using Piezo-ICSI. We predict that this also helps to reduce physical damage to the oocyte. Therefore, it is suggested that the Piezo-ICSI is clinically useful for improving the survival and fertilization rates for human oocytes.
The rates of survival and the fertilization using CPI in the present study (survival rate 95 %, fertilization rate 75 %) are not superior to those recently reported using conventional ICSI (survival rate 89–93 %, fertilization rate 62–77 %) [10–14]; it may be possible that the current protocol of the Piezo-ICSI using micropipettes with a wall thickness of 0.925 μm is not the most suitable for human oocytes. Therefore, in this study, we designed a new Piezo-ICSI method which used micropipettes with a wall thickness of 0.625 μm. To the best of our knowledge, there are no reports evaluating the wall thickness of the micropipette (0.925 vs 0.625 μm) in regard to the survival and fertilization rates after Piezo-ICSI for human oocytes. In the present study, significantly higher survival and fertilization rates were observed using IPI as compared to the CPI (survival rates 95 vs 99 %, fertilization rates 75 vs 89 %). The injection sites in the membrane of CPI using micropipettes with a wall thickness of 0.925 μm and IPI using micropipettes with a wall thickness of 0.625 μm were 17 and 11 μm2, respectively. It is suggested that the physical damage to the oocyte was reduced by creating a smaller injection site (17 vs 11 μm2); this smaller injection site could be one of the factors explaining why the survival and fertilization rates of IPI were significantly higher as compared to the CPI.
Good-quality day-3 embryo rates were found to be significantly higher (55 %) when using IPI as compared to those of CI (37 %) and CPI (43 %). Furthermore, the pregnancy, implantation, and live birth rates were found to be significantly higher (31, 31, and 25 %) when using IPI as compared to those of CI (19, 19, and 15 %) and CPI (21, 21, and 16 %). Consequently, we suggest that the IPI can significantly improve the effective utilization rate of injected oocytes and can contribute to increasing live birth rates.
In the present study, the effect of micropipette wall thickness of 0.925 vs 0.625 μm on the clinical results was assessed only when using Piezo-ICSI, but not using CI method. Since the inner diameter of the micropipette for the CI is the same as the inner diameter for both CPI and IPI methods (5 μm), the wall thickness will not affect the volume of cytoplasm aspirated into the micropipette in the CI method.
In conclusion, the Piezo-ICSI using micropipettes with a wall thickness of 0.625 μm should be a less damaging ICSI procedure for the oocyte, and this could be useful for general infertility patients by improving the survival and fertilization rates. It could also be beneficial for hematological cancer patients who need oocyte cryopreservation or for female patients of advanced age whose oocyte membranes are more likely to be weak.
Acknowledgments
We are indebted to Ms. Adair Oesterle for her critical reading of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Capsule Piezo-ICSI, in combination with flat-tipped micropipettes with a wall thickness of 0.625 μm, significantly improves survival, fertilization, good-quality day-3 embryo, pregnancy, and live birth rates as compared to conventional ICSI.
References
- 1.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 2.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–20. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 3.Huang T, Kimura Y, Yanagimachi R. The use of piezo micromanipulation for intracytoplasmic sperm injection of human oocytes. J Assist Reprod Genet. 1996;13:320–8. doi: 10.1007/BF02070146. [DOI] [PubMed] [Google Scholar]
- 4.Yanagida K, Katayose H, Yazawa H, Kimura Y, Konnai K, Sato A. The usefulness of a piezo-micromanipulator in intracytoplasmic sperm injection in humans. Hum Reprod. 1999;14:448–53. doi: 10.1093/humrep/14.2.448. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi S, Minoura H, Shibahara T, Shen X, Futamura N, Toyoda N. Comparison of piezo-assisted micromanipulation with conventional micromanipulation for intracytoplasmic sperm injection into human oocytes. Gynecol Obstet Investig. 2001;52:158–62. doi: 10.1159/000052965. [DOI] [PubMed] [Google Scholar]
- 6.Ferraretti AP, Gianaroli L, Magli MC, Devroey P. Mild ovarian stimulation with clomiphene citrate launch is a realistic option for in vitro fertilization. Fertil Steril. 2015 doi: 10.1016/j.fertnstert.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Diedrich K, Diedrich C, Santos E, Zoll C, al-Hasani S, Reissmann T, et al. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod. 1994;9(5):788–91. doi: 10.1093/oxfordjournals.humrep.a138597. [DOI] [PubMed] [Google Scholar]
- 8.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–14. doi: 10.1016/S1472-6483(10)61169-8. [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka K, Hiraoka K, Horiuchi T, Kusuda T, Okano S, Kinutani M, et al. Impact of the size of zona pellucida thinning area on vitrified-warmed cleavage-stage embryo transfers: a prospective, randomized study. J Assist Reprod Genet. 2009;26:515–21. doi: 10.1007/s10815-009-9350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumoulin JM, Coonen E, Bras M, Bergers-Janssen JM, Ignoul-Vanvuchelen RC, van Wissen LC, et al. Embryo development and chromosomal anomalies after ICSI: effect of the injection procedure. Hum Reprod. 2001;16:306–12. doi: 10.1093/humrep/16.2.306. [DOI] [PubMed] [Google Scholar]
- 11.Abdelmassih S, Cardoso J, Abdelmassih V, Dias JA, Abdelmassih R, Nagy ZP. Laser-assisted ICSI: a novel approach to obtain higher oocyte survival and embryo quality rates. Hum Reprod. 2002;17:2694–9. doi: 10.1093/humrep/17.10.2694. [DOI] [PubMed] [Google Scholar]
- 12.Ebner T, Moser M, Sommergruber M, Jesacher K, Tews G. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod. 2004;19:1837–41. doi: 10.1093/humrep/deh325. [DOI] [PubMed] [Google Scholar]
- 13.Richter KS, Davis A, Carter J, Greenhouse SJ, Mottla GL, Tucker MJ. No advantage of laser-assisted over conventional intracytoplasmic sperm injection: a randomized controlled trial. J Exp Clin Assist Reprod. 2006;3:5. doi: 10.1186/1743-1050-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vos A, Van Landuyt L, Van Ranst H, Vandermonde A, D’Haese V, Sterckx J, et al. Randomized sibling-oocyte study using recombinant human hyaluronidase versus bovine-derived Sigma hyaluronidase in ICSI patients. Hum Reprod. 2008;23:1815–9. doi: 10.1093/humrep/den212. [DOI] [PubMed] [Google Scholar]