Abstract
Purpose
The purpose of the present study is to determine if paternal or maternal history of diabetes mellitus (DM) and hypertension (HT) contributes to the prevalence and phenotype of polycystic ovary syndrome (PCOS).
Methods
We performed an epidemiologic study about PCOS from four districts in Beijing, China, between 2008 and 2009. Parental histories of DM and HT were collected, and the basic characteristics and serum indices of 123 PCOS patients and 718 non-PCOS controls were tested.
Results
The prevalence of a parental history of DM and HT was significantly higher in PCOS patients than non-PCOS women (17.1 % vs. 9.2 % and 42.3 % vs. 26.0 %, P < 0.05, respectively). When paternal history was separated from maternal history, only a paternal history of DM and HT reached statistical significance between PCOS and non-PCOS patients (odds ratio (OR) = 3.42, 95 % confidence interval (CI) = 1.69–6.91; OR = 2.50, 95 % CI = 1.58–3.93, respectively). A paternal history of both DM and HT was significantly associated with sex hormone-binding globulin, fasting plasma glucose, and fasting insulin levels, the free androgen index, and the homeostatic model assessment-insulin resistance in PCOS patients (P < 0.05 for all). There was no independent association between maternal history and the clinical or biochemical phenotype of PCOS.
Conclusions
PCOS patients with a positive paternal history of both DM and HT have an adverse endocrine and metabolic profile. A paternal history of DM and HT poses a risk to PCOS.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0587-y) contains supplementary material, which is available to authorized users.
Keywords: Polycystic ovary syndrome (PCOS), Paternal history, Diabetes mellitus, Hypertension, Phenotype
Introduction
Polycystic ovary syndrome (PCOS) is a common reproductive, endocrine, and metabolic disorder characterized by oligoanovulation, hyperandrogenism, insulin resistance, and polycystic morphology of the ovary, which accounts for 5–10 % of women of reproductive age [1, 2]. Since first described by Stein and Leventhal in 1935 [3], PCOS has gained special interest in clinical and basic research; however, we still know little about the pathogenesis of PCOS. An abundance of evidence has confirmed that there is a familial clustering of PCOS [4–6]. Numerous candidate genes have been reported to be associated with obesity, elevated testosterone levels, and the metabolic disturbance in PCOS patients [7–10].
Insulin resistance (IR) is common in PCOS patients and plays an essential role in the pathophysiology of endocrine and metabolic complications of PCOS patients. It has been suggested that IR contributes to PCOS and an increased risk of metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM), and cardiovascular disease [11]. Furthermore, evidence has shown that a family history of diabetes mellitus (DM) and MetS is significantly higher among women with PCOS and strongly associated with an increased risk of DM and metabolic disorders in women with PCOS [12]. Lerchbaum et al. [13] reported that a positive family history of DM was independently associated with metabolic disorders, while a positive family history of PCOS was independently associated with hyperandrogenism. Kulshreshtha et al. [14] reported that the number of parents with hypertension (HT) and DM is associated with the initial symptoms of PCOS. No additional studies separated a paternal history of DM and HT from a maternal history of DM and HT. The aim of our study was to determine if a paternal or maternal history of DM and HT contributed to the prevalence and phenotype of PCOS.
Materials and methods
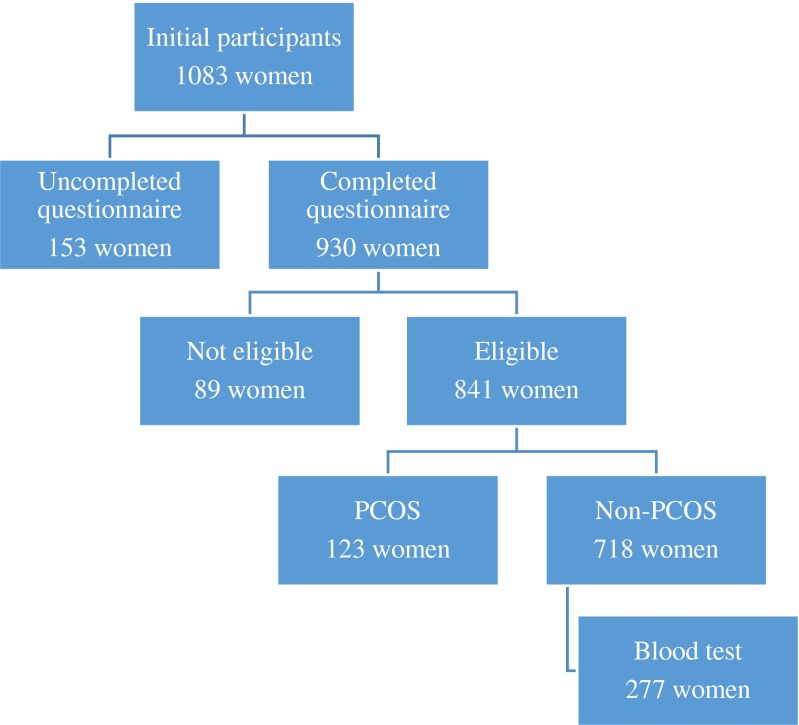
We performed an epidemiologic study involving PCOS patients from four districts in Beijing, China, between 2008 and 2009. A total of 1083 women were involved in the study, and 930 women completed the questionnaire. Only reproductive-aged women (21–45 years of age) were enrolled in the final data analysis. Women who took any medications affecting endocrine and metabolic parameters were ruled out (Fig. 1). The study was approved by the Ethics Committee of Peking University Third Hospital, and written informed consent was obtained from all participants.
Fig. 1.
Flowchart of participants for the study involving the prevalence of PCOS in Beijing
A detailed history was obtained from all participants, and a complete physical examination was performed, including age, height, weight, waist and hip circumferences, blood pressure, menstrual cycle characteristics, pelvic ultrasonography, and serum indices. Parental histories were collected through questionnaires. The questions were arranged as follows: parental history of DM and/or HT (yes or no; father or mother). In addition, information was collected with respect to histories of gynecologic tumors, infertility, and oligomenorrhea in the mother and a history of premature alopecia in the father. Parental history was considered positive if the father or mother was diagnosed by specialists and on medications; otherwise, the history was recorded as negative. The body mass index (BMI) was calculated as the weight / height2. Central obesity was calculated as the waist–hip ratio (WHR). Transvaginal ultrasonography was performed to detect the volumes of the uterus and ovary and the number of antral follicles. Polycystic ovary (PCO) was defined as >12 follicles in each ovary, measuring 2–9 mm in diameter, and/or >10-mL ovarian volume bilaterally [15].
Among the non-PCOS women, 277 were selected based on blood testing as normal controls who had no clinical evidence of hyperandrogenism and were not taking any hormonal medications. Blood samples were collected in the morning after fasting for >8 h. Total testosterone (TT), androstenedione (A), sex hormone-binding globulin (SHBG), and fasting insulin (FI) were tested by chemiluminescence (intra- and inter-assay coefficients of variation <10 %; DPC, USA). Serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels were determined using a dry-slide enzymatic colorimetric assay. The fasting plasma glucose (FPG) level was tested using the finger stick blood glucose method (Roche ACCU-Chek). IR was determined by the homeostatic model assessment (HOMA-IR = fasting glucose × fasting insulin / 22.5). The free androgen index (FAI) was defined as TT × 100 / SHBG.
The diagnosis of PCOS was based on the 2003 Rotterdam consensus [16], containing at least two of the following three characteristics: (1) oligoovulation or anovulation, (2) clinical and/or biochemical signs of hyperandrogenism, and (3) polycystic ovaries. In addition, other etiologies (congenital adrenal hyperplasia, androgen-secreting tumors, and Cushing’s syndrome) were excluded.
Metabolic syndrome was diagnosed according to the NCEP ATP III guidelines [17]. MetS was diagnosed if three or more of the following five findings were present: (1) high blood pressure ≥130/85 mmHg or anti-hypertensive drugs prescribed, (2) hypertriglyceridemia (TG ≥1.7 mmol/L, (3) low HDL (<1.3 mmol/L), (4) high FPG (≥5.6 mmol/L), and (5) central obesity (waist circumference ≥80 cm for Chinese).
Statistical analysis
All data were recorded and analyzed by SPSS software (version 19; SPSS, Inc., Chicago, IL, USA). Categorical variables were presented as proportions and analyzed by the chi-square test or Fisher’s exact test, as appropriate. The normality of continuous variables was analyzed by the Kolmogorov–Smirnov test or analysis of variance (ANOVA). Variables of normal distribution were presented as the mean ± standard deviation (SD) and analyzed by an independent sample t test or ANOVA. Variables of non-normal distribution were presented as the median (interquartile range) and tested by the Mann–Whitney U test or Kruskal–Wallis H test. Binary logistic regression and linear regression analyses were used to examine independent predictors. Statistical significance was considered present if the P value was <0.05.
Results
There were 841 women eligible for the study (123 PCOS patients and 718 non-PCOS controls). All PCOS and 277 non-PCOS patients underwent blood testing. The demographic data are presented in Table 1. The PCOS group had a younger age, earlier age of menarche, more rapid heart rate, smaller uterine volume, larger ovarian volume, and significantly greater antral follicle count. With respect to blood test results, the PCOS group had higher levels of TT and A and lower levels of SHBG and HDL compared with the control group. With respect to menstrual status, 54.5 and 6.9 % of PCOS patients and controls had cycle disorders, respectively. There was no significant difference in menstrual duration, menstrual volume, and dysmenorrhea.
Table 1.
The baseline characteristics of PCOS and non-PCOS patients
| PCOS (n = 123) | non-PCOS (n = 718) | P value | |
|---|---|---|---|
| Age (years) | 31 (27–35) | 37 (31–40) | <0.0001* |
| Menarche (years) | 14 (13–16) | 15 (14–16) | 0.009* |
| BMI (kg/m2) | 22 (20–26) | 23 (21–26) | 0.297 |
| WHR | 0.83 ± 0.06 | 0.82 ± 0.06 | 0.084 |
| SBP (mmHg) | 115 (110–120) | 110 (110–120) | 0.171 |
| DBP (mmHg) | 80 (70–80) | 74 (70–80) | 0.626 |
| HR (bpm) | 78 (75–80) | 76 (74–80) | 0.044* |
| Uterine volume (cm3) | 83.03 (68.80–99.84) | 97.02 (76.52–121.64) | <0.0001* |
| Endometrial thickness (mm) | 8 (7–10) | 9 (7–10) | 0.717 |
| L-ovarian volume (cm3) | 10.40 (7.14–13.67) | 5.89 (4.02–8.77) | <0.0001* |
| L-AFC | 12 (9–15) | 4 (3–6) | <0.0001* |
| R-ovarian volume (cm3) | 9.54 (7.05–14.03) | 6.40 (4.12–9.75) | <0.0001* |
| R-AFC | 12 (8–15) | 4 (3–6) | <0.0001* |
| TT (nmol/L) | 1.61 (1.03–2.24) | 0.87 (0.69–1.20) | <0.0001* |
| A (nmol/L) | 13.30 ± 4.42 | 7.28 ± 2.32 | <0.0001* |
| SHBG (nmol/L) | 46.39 ± 23.44 | 59.59 ± 28.37 | <0.0001* |
| FPG (mmol/L) | 4.80 (4.50–5.43) | 5.00 (4.58–5.51) | 0.563 |
| FI (IU/L) | 4.86 (2.41–11.35) | 3.97 (2.00–7.71) | 0.096 |
| TG (mmol/L) | 1.08 (0.80–1.90) | 1.04 (0.79–1.50) | 0.245 |
| TC (nmol/L) | 4.37 ± 1.22 | 4.45 ± 0.90 | 0.525 |
| HDL (nmol/L) | 1.19 (0.96–1.40) | 1.28 (1.12–1.48) | 0.005* |
| LDL (nmol/L) | 2.12 (1.67–2.54) | 2.09 (1.77–2.48) | 0.869 |
| FAI | 3.62 (2.16–6.46) | 1.72 (1.12–2.70) | <0.0001* |
| HOMA-IR | 1.34 (0.62–3.26) | 1.17 (0.56–2.18) | 0.340 |
| MetS (%) | 31.8 (28/88) | 24.2 (38/157) | 0.197 |
| Menstrual irregularities (%) | 54.5 (67/123) | 6.9 (49/714) | <0.0001* |
| Dysmenorrhea (%) | 37.4 (46/123) | 38.2 (271/710) | 0.871 |
Data are presented as the median (interquartile range), mean ± SD, or proportion (%, n/N)
PCOS polycystic ovary syndrome, BMI body mass index, WHR waist-to-hip ratio, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, L-AFC left antral follicle count, R-AFC right antral follicle count, TT total testosterone, A androstenedione, SHBG sex hormone-binding globulin, FPG fasting plasma glucose, FI fasting insulin, TG triglycerides, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, FAI free androgen index, HOMA-IR homeostatic model assessment-insulin resistance, MetS metabolic syndrome
*Refers to statistical difference
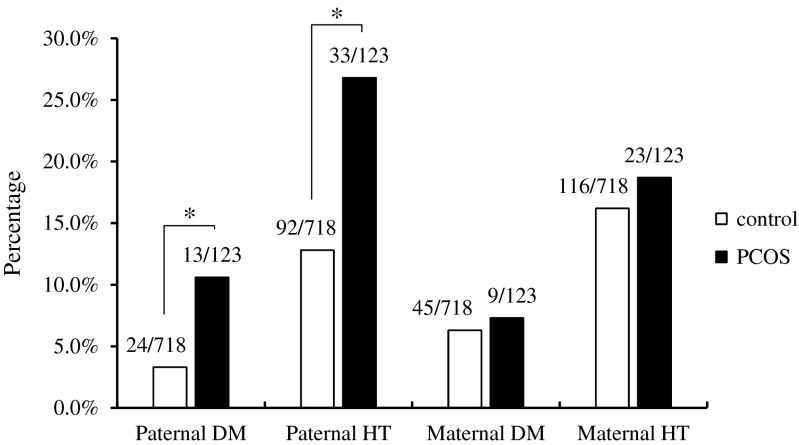
The prevalence of parental histories is shown in Supplement 1. There was an increased prevalence of parental DM histories in the PCOS group compared to the control group (17.1 vs. 9.2 %, odds ratio (OR) = 2.03, 95 % confidence interval (CI) = 1.19–3.47, P = 0.008). The prevalence of parental histories of HT was more frequent in PCOS patients than non-PCOS controls (42.3 vs. 26.0 %, OR = 2.08, 95 % CI = 1.40–3.09, P < 0.0001). Additionally, parental histories of gynecologic tumors, oligomenorrhea, and premature alopecia were more common in the PCOS group. Interestingly, when paternal history was separated from maternal history, the only significant difference between PCOS and non-PCOS patients involved paternal DM and HT histories (10.6 vs. 3.3 %, OR = 3.42, 95 % CI = 1.69–6.91 and 26.8 vs. 12.8 %, OR = 2.50, 95 % CI = 1.58–3.93, respectively); there was no significant difference in maternal DM and HT histories between the groups (7.3 vs. 6.3 %, OR = 1.18, 95 % CI = 0.56–2.48 and 18.7 vs. 16.2 %, OR = 1.19, 95 % CI = 0.73–1.96, respectively; Fig. 2). Moreover, binary logistic regression analyses confirmed that paternal DM and HT histories are independently associated with the prevalence of PCOS adjusted for other family medical histories (OR = 2.62, 95 % CI = 1.23–5.57 and OR = 1.97, 95 % CI = 1.20–3.24, respectively).
Fig. 2.
The prevalence of a parental history of DM and HT in PCOS patients and the controls. Data are presented as n/N and analyzed by chi-square test/Fisher’s exact test. Asterisk refers to statistic difference; DM diabetes mellitus, HT hypertension
To determine whether or not a paternal history of DM and HT affects the phenotype of PCOS, we divided PCOS patients into two groups based on a positive versus negative paternal history. As shown in Table 2, the serum level of SHBG was significantly lower in the exposure group than the negative group (38.10 vs. 50.92, P = 0.004). Therefore, the calculated FAI was significantly higher in PCOS patients with a positive paternal history than the negative paternal history group (4.66 vs. 3.35, P = 0.022). The BMI, WHR, FPG, lipid parameters, and prevalence of MetS and irregular menstruation were comparable within the groups. When a paternal DM history was separated from a paternal HT history, a lower SHBG level was noted in the positive paternal DM and positive paternal HT history groups. In addition, a higher FAI was shown in the positive paternal DM history group than the negative paternal DM history group (Supplement 2 and 3, respectively). When dividing PCOS patients based on a positive versus negative maternal history, only a lower A level was noted in the exposure group; the other indices did not differ significantly between the groups (Table 3). Linear regression analysis showed that SHBG and FAI were significantly related to paternal DM and HT histories after correcting for age, BMI, and other family history (Table 4).
Table 2.
Characteristics of PCOS patients stratified by paternal history of DM and HT
| Positive paternal history (n = 41) | Negative paternal history (n = 82) | P value | |
|---|---|---|---|
| Age (years) | 32.10 ± 5.03 | 30.79 ± 5.30 | 0.196 |
| Menarche (years) | 14.15 ± 1.54 | 14.44 ± 1.71 | 0.357 |
| BMI (kg/m2) | 24 (21–27) | 22 (20–26) | 0.732 |
| WHR | 0.83 ± 0.05 | 0.83 ± 0.06 | 0.530 |
| TT (nmol/L) | 1.83 ± 0.97 | 1.72 ± 0.85 | 0.542 |
| A (nmol/L) | 13.53 ± 4.99 | 13.17 ± 4.10 | 0.674 |
| SHBG (nmol/L) | 38.10 ± 19.89 | 50.92 ± 24.11 | 0.004* |
| FPG (mmol/L) | 5.10 ± 1.05 | 4.98 ± 1.02 | 0.652 |
| FI (IU/L) | 4.99 (2.71–7.61) | 4.25 (2.28–12.30) | 0.917 |
| TG (mmol/L) | 1.30 (0.90–2.20) | 0.96 (0.71–1.73) | 0.061 |
| TC (nmol/L) | 4.54 ± 0.99 | 4.29 ± 1.32 | 0.286 |
| HDL (nmol/L) | 1.21 (1.02–1.53) | 1.16 (0.96–1.39) | 0.178 |
| LDL (nmol/L) | 2.19 ± 0.70 | 2.18 ± 0.84 | 0.948 |
| FAI | 4.66 (2.91–9.58) | 3.35 (1.82–6.20) | 0.022* |
| HOMA-IR | 1.31 (0.90–1.50) | 1.56 (0.51–4.01) | 0.577 |
| MetS (%) | 36.4 (12/33) | 29.1 (16/55) | 0.478 |
| Menstrual irregularities (%) | 58.5 (24/41) | 52.4 (43/82) | 0.522 |
Data are presented as the median (interquartile range), mean ± SD, or proportion (%, n/N)
DM diabetes mellitus, HT hypertension, BMI body mass index, WHR waist-to-hip ratio, TT total testosterone, A androstenedione, SHBG sex hormone-binding globulin, FPG fasting plasma glucose, FI fasting insulin, TG triglycerides, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, FAI free androgen index, HOMA-IR homeostatic model assessment-insulin resistance, MetS metabolic syndrome
*Refers to statistical difference
Table 3.
Characteristics of PCOS patients stratified by maternal history of DM and HT
| Positive maternal history (n = 29) | Negative maternal history (n = 94) | P value | |
|---|---|---|---|
| Age (years) | 31.62 ± 5.72 | 31.10 ± 5.09 | 0.639 |
| Menarche (years) | 13.86 ± 1.62 | 14.49 ± 1.64 | 0.074 |
| BMI (kg/m2) | 24 (20–27) | 22 (20–25) | 0.162 |
| WHR | 0.84 ± 0.05 | 0.83 ± 0.06 | 0.338 |
| TT (nmol/L) | 1.74 ± 0.74 | 1.76 ± 0.94 | 0.886 |
| A (nmol/L) | 12.03 ± 3.16 | 13.70 ± 4.69 | 0.035* |
| SHBG (nmol/L) | 42.88 ± 23.57 | 47.51 ± 23.43 | 0.365 |
| FPG (mmol/L) | 5.01 ± 0.82 | 5.02 ± 1.07 | 0.973 |
| FI (IU/L) | 3.26 (2.00–14.95) | 5.12 (2.61–10.25) | 0.338 |
| TG (mmol/L) | 1.25 (0.85–2.16) | 1.08 (0.78–1.85) | 0.689 |
| TC (nmol/L) | 4.42 ± 1.32 | 4.36 ± 1.19 | 0.833 |
| HDL (nmol/L) | 1.20 ± 0.35 | 1.24 ± 0.47 | 0.656 |
| LDL (nmol/L) | 2.27 ± 0.92 | 2.15 ± 0.75 | 0.476 |
| FAI | 4.31 (2.19–9.13) | 3.62 (2.01–6.18) | 0.412 |
| HOMA-IR | 1.28 (0.44–3.74) | 1.43 (0.63–3.15) | 0.808 |
| MetS (%) | 36.4 (8/22) | 30.3 (20/66) | 0.597 |
| Menstrual irregularities (%) | 65.5 (19/29) | 51.1 (48/94) | 0.172 |
Data are presented as the median (interquartile range), mean ± SD, or proportion (%, n/N)
DM diabetes mellitus, HT hypertension, BMI body mass index, WHR waist-to-hip ratio, TT total testosterone, A androstenedione, SHBG sex hormone-binding globulin, FPG fasting plasma glucose, FI fasting insulin, TG triglycerides, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, FAI free androgen index, HOMA-IR homeostatic model assessment-insulin resistance, MetS metabolic syndrome
*Refers to statistical difference
Table 4.
Linear logistic regression analyses of SHBG and FAI using parental history of DM and HT as explanatory variables adjusting for age, BMI, and other family history
| Paternal DM | Paternal HT | Maternal DM | Maternal HT | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | P value | OR (95 % CI) | P value | OR (95 % CI) | P value | OR (95 % CI) | P value | |
| SHBG | −15.34 (−27.47 to −3.21) | 0.014* | −11.75 (−20.68 to −2.81) | 0.010* | −5.06 (−19.15–9.04) | 0.478 | 1.70 (−8.14–11.53) | 0.733 |
| FAI | 5.14 (2.46–7.82) | <0.0001* | 2.61 (0.63–4.59) | 0.010* | −0.25 (−3.37–2.87) | 0.873 | 0.21 (−1.97–2.38) | 0.851 |
SHBG sex hormone-binding globulin, FAI free androgen index, DM diabetes mellitus, HT hypertension
*Refers to statistical difference
We conducted further analyses that divided PCOS patients into three groups based on the number of paternal diagnosed diseases. Patients were assigned to group 0 if there was no paternal history of DM or HT, group 1 if there was a paternal history of DM or HT, or group 2 if there was a paternal history of both DM and HT. An increasing trend of FPG, FI, FAI, and HOMA-IR was demonstrated in group 2 (Table 5); a decrease of SHBG was observed in group 2. Endocrine and metabolic homeostasis was decreased with the increase in the number of affected paternal diseases. While we also divided PCOS patients based on the number of affected maternal diseases, no significant difference was observed between groups except a lower A level in group 1 compared to group 0 (Table 6).
Table 5.
Characteristics of PCOS patients stratified by the number of fathers diagnosed with diseases
| 0 (n = 82) | 1 (n = 36) | 2 (n = 5) | P value | |
|---|---|---|---|---|
| Age (years) | 30.79 ± 5.30 | 32.23 ± 5.19 | 31.20 ± 4.09 | 0.399 |
| Menarche (years) | 14.44 ± 1.71 | 14.03 ± 1.48 | 15.00 ± 1.87 | 0.308 |
| BMI (kg/m2) | 23.48 ± 4.33 | 23.47 ± 3.61 | 22.80 ± 2.77 | 0.937 |
| WHR | 0.83 ± 0.06 | 0.83 ± 0.05 | 0.83 ± 0.05 | 0.820 |
| TT (nmol/L) | 1.72 ± 0.85 | 1.84 ± 1.00 | 1.72 ± 0.86 | 0.796 |
| A (nmol/L) | 13.17 ± 4.10 | 13.65 ± 5.17 | 12.70 ± 3.78 | 0.829 |
| SHBG (nmol/L) | 50.92 ± 24.11 | 40.37 ± 19.84 | 21.72 ± 11.45 | 0.004*† |
| FPG (mmol/L) | 4.98 ± 1.02 | 4.96 ± 0.83 | 6.50 ± 2.40 | 0.041†‡ |
| FI (IU/L) | 10.76 ± 14.52 | 7.04 ± 7.24 | 27.11 ± 50.83 | 0.028†‡ |
| TG (mmol/L) | 1.32 ± 0.83 | 1.56 ± 0.88 | 1.68 ± 1.18 | 0.298 |
| TC (nmol/L) | 4.29 ± 1.32 | 4.47 ± 0.86 | 5.01 ± 1.71 | 0.370 |
| HDL (nmol/L) | 1.21 ± 0.49 | 1.26 ± 0.33 | 1.39 ± 0.33 | 0.627 |
| LDL (nmol/L) | 2.18 ± 0.84 | 2.13 ± 0.65 | 2.61 ± 1.00 | 0.442 |
| FAI | 3.35 (1.82–6.20) | 4.58 (2.93–9.21) | 6.54 (2.91–27.20) | 0.050†‡ |
| HOMA-IR | 3.61 ± 4.78 | 1.95 ± 2.36 | 7.83 ± 4.05 | 0.031c‡ |
| MetS (%) | 29.1 (16/55) | 36.7 (11/30) | 33.3 (1/3) | 0.772 |
| Menstrual irregularities (%) | 52.4 (43/82) | 58.3 (21/36) | 60.0 (3/5) | 0.813 |
Patients were assigned to group 0 if there was no paternal history of DM or HT, group 1 if there was a paternal history of either DM or HT, or group 2 if there was a paternal history of DM and HT. Data are presented as the median (interquartile range), mean ± SD, or proportion (%, n/N)
BMI body mass index, WHR waist-to-hip ratio, TT total testosterone, A androstenedione, SHBG sex hormone-binding globulin, FPG fasting plasma glucose, FI fasting insulin, TG triglycerides, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, FAI free androgen index, HOMA-IR homeostatic model assessment-insulin resistance, MetS metabolic syndrome
*P < 0.05 for group 0 versus group 1 (SLD and Student–Newman–Keuls test); †P < 0.05 for group 0 versus group 2 (SLD and Student–Newman–Keuls test); ‡P < 0.05 for group 1 versus group 2 (SLD and Student–Newman–Keuls test)
Table 6.
Characteristics of PCOS patients stratified by the number of mothers diagnosed with diseases
| 0 (n = 94) | 1 (n = 26) | 2 (n = 3) | P value | |
|---|---|---|---|---|
| Age (years) | 31.10 ± 5.09 | 31.15 ± 5.84 | 35.67 ± 2.08 | 0.331 |
| Menarche (years) | 14.49 ± 1.64 | 13.92 ± 1.70 | 13.33 ± 0.58 | 0.172 |
| BMI (kg/m2) | 22 (20–25) | 24 (20–27) | 26 (24–27) | 0.218 |
| WHR | 0.83 ± 0.06 | 0.84 ± 0.05 | 0.86 ± 0.06 | 0.547 |
| TT (nmol/L) | 1.76 ± 0.94 | 1.68 ± 0.75 | 2.17 ± 0.56 | 0.668 |
| A (nmol/L) | 13.70 ± 4.69 | 11.69 ± 3.07 | 14.83 ± 2.92 | 0.044* |
| SHBG (nmol/L) | 47.51 ± 23.43 | 44.67 ± 24.29 | 27.93 ± 6.43 | 0.337 |
| FPG (mmol/L) | 5.02 ± 1.07 | 5.01 ± 0.86 | 5.00 ± 1.00 | 0.999 |
| FI (IU/L) | 10.33 ± 16.37 | 10.59 ± 16.01 | 7.40 ± 7.19 | 0.949 |
| TG (mmol/L) | 1.40 ± 0.86 | 1.51 ± 0.91 | 0.93 ± 0.09 | 0.536 |
| TC (nmol/L) | 4.36 ± 1.19 | 4.40 ± 1.40 | 4.56 ± 0.26 | 0.956 |
| HDL (nmol/L) | 1.24 ± 0.47 | 1.21 ± 0.37 | 1.09 ± 0.16 | 0.817 |
| LDL (nmol/L) | 2.15 ± 0.75 | 2.21 ± 0.09 | 2.77 ± 0.79 | 0.399 |
| FAI | 5.09 ± 5.02 | 5.62 ± 4.66 | 8.45 ± 4.44 | 0.478 |
| HOMA-IR | 2.90 ± 4.05 | 3.14 ± 4.49 | 3.49 ± 4.01 | 0.978 |
| MetS (%) | 30.3 (20/66) | 36.8 (7/19) | 33.3 (1/3) | 0.863 |
| Menstrual irregularities (%) | 51.1 (48/94) | 69.2 (18/26) | 33.3 (1/3) | 0.195 |
Patients were assigned to group 0 if there was no maternal history of DM or HT, group 1 if there was a maternal history of DM or HT, or group 2 if there was a maternal history of DM and HT. Data are presented as the median (interquartile range), mean ± SD, or proportion (%, n/N)
BMI body mass index, WHR waist-to-hip ratio, TT total testosterone, A androstenedione, SHBG sex hormone-binding globulin, FPG fasting plasma glucose, FI fasting insulin, TG triglycerides, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, FAI free androgen index, HOMA-IR homeostatic model assessment-insulin resistance, MetS metabolic syndrome
*P < 0.05 for group 0 versus group 1 (SLD and Student–Newman–Keuls test)
Discussion
In the current study, we investigated all of the reproductive-aged women from four districts in Beijing. The PCOS patients presented with irregular menstruation, PCO morphology, androgen excess, and an increased prevalence of endocrine and metabolic disorders, features which were consistent with the characteristics of PCOS according to international consensus. The PCOS group is known to be at a higher risk for long-term complications of coronary heart disease (CHD), DM, and MetS. Elevated heart rate is an early marker of myocardial ischemia [18]. An anti-atherogenic lipoprotein, HDL, plays an important role in reverse cholesterol transport from tissues to liver and has been associated with a lower risk of CHD and MetS [19]. Larger ovarian volume and smaller uterine volume were observed in PCOS patients compared to the controls. Eryilmaz et al. [20] showed that the right and left ovarian volumes were significantly greater in PCOS patients than non-PCOS patients. With the use of a 9-MHz transvaginal transducer and Santesoft DICOM Editor Software to analyze ultrasound images, Christ et al. [21] reported that the antral follicle count, rather than the ovarian volume, reflected the severity of reproductive and metabolic disturbance in PCOS patients. Few publications involving uterine volume in PCOS patients exist [22].
Familial clustering of PCOS is well documented [23, 24]. An increased prevalence of DM and HT was observed among the parents of PCOS patients compared to non-PCOS controls in our study. More specifically, a paternal history of DM and HT was associated with the prevalence of PCOS. The morbidity of maternal DM and HT did not reach statistical significance between the groups. It appeared that a paternal history of DM and HT was a risk factor for PCOS. Kulshreshtha et al. [14] obtained the same result; specifically, a paternal history of HT is more common among PCOS patients than controls, while a maternal history of HT did not confer an increased risk for PCOS. Moini et al. [25] reported a significantly increased prevalence of maternal DM in the PCOS group than the control group, and a maternal history of DM was more predictive than a paternal history of DM. Moreover, mothers of women with PCOS had a higher HOMA-IR, an increased prevalence of MetS, and an elevated risk for CHD [26]. Whether paternal or maternal inheritance underlies PCOS has been a matter of controversy and varies in different populations and races.
Our results indicated that paternal history had a greater influence on PCOS phenotype than maternal history. A paternal history of DM and HT was shown to be independently associated with SHBG and FAI, excluding the impact of additional parental history. In contrast, maternal history was only related to the serum A level and showed no significant association with SHBG, HOMA-IR, and other recorded metabolic parameters. The prevalence of gynecologic tumors, oligomenorrhea, infertility, and premature alopecia was not different between the positive and negative history groups and had no effect on TT, SHBG, FAI, and metabolic parameters (data not shown). Our assessment and questionnaire were not comprehensive, and other confounding factors may not have been considered, such as the age of onset, severity of parental DM and/or HT, and history of CHD and MetS. SHBG was significantly lower in the positive paternal history group. SHBG is a protective factor, and a low level of SHBG might serve as a critical factor during the pathogenesis of PCOS and MetS [27]. Several sources of evidence have shown that decreased SHBG is associated with MetS and IR in women with PCOS [28–32]. Therefore, paternal history aggravates the metabolic disturbance in PCOS patients. These findings might have implications for clinical practice. Those PCOS patients with a positive paternal history of DM and HT might be at high risk for metabolic disorders. Risk stratification of PCOS patients should be done, and early prevention and treatment should be implemented.
Interestingly, our results showed an increased FAI and HOMA-IR in PCOS patients with a positive paternal history of both DM and HT, while this trend was not observed in the positive maternal history group. The third ESHRE/ASRM consensus [33] pointed out that metabolic disorders of PCOS are predictors of DM and MetS, and not all PCOS phenotypes have a similar metabolic risk. To stratify PCOS patients according to metabolic risk was suggested. We could discreetly define this subset of PCOS patients with a positive paternal history of both DM and HT as a high-risk group with an increased prevalence of endocrine and metabolic disturbances.
We separated paternal history from maternal history of PCOS patients, and it showed a different influence on the prevalence and phenotype of PCOS. The limitation of our study was that we collected the family history via questionnaires, but we did not confirm the diagnosis via medical testing. It was possible that the use of questionnaires might impair the reliability of our findings.
In conclusion, the prevalence of a parental history of DM and HT was significantly higher in women with PCOS than non-PCOS controls. A paternal history of DM and HT contributed more risk to PCOS. Not only the prevalence of PCOS, but also the phenotype of PCOS was affected by a paternal history of DM and HT. PCOS patients with a positive paternal history of both DM and HT had a higher FAI and HOMA-IR. We have thus defined PCOS women with a positive paternal history of both DM and HT as a high-risk group with an increased prevalence of endocrine and metabolic disturbances, and a parental history should be collected in clinical practice.
Electronic supplementary material
(DOC 31 kb)
(DOCX 18 kb)
(DOCX 18 kb)
Acknowledgments
This study was supported by the National Key Technology R&D Program (grant number: 2007BAI04B04 and 2012BAI32B01).
Footnotes
Capsule We define a subset of PCOS patients with a positive paternal history of both DM and HT as a high-risk group with an increased prevalence of endocrine and metabolic disturbances.
References
- 1.Kollmann M, Klaritsch P, Martins WP, Guenther F, Schneider V, Herzog SA, et al. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum Reprod. 2015. [DOI] [PubMed]
- 2.Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28:2562–2569. doi: 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]
- 3.Stein IL, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. doi: 10.1016/S0002-9378(15)30642-6. [DOI] [Google Scholar]
- 4.Lunde O, Magnus P, Sandvik L, Hoglo S. Familial clustering in the polycystic ovarian syndrome. Gynecol Obstet Investig. 1989;28:23–30. doi: 10.1159/000293493. [DOI] [PubMed] [Google Scholar]
- 5.Strauss JR, McAllister JM, Urbanek M. Persistence pays off for PCOS gene prospectors. J Clin Endocrinol Metab. 2012;97:2286–2288. doi: 10.1210/jc.2012-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAllister JM, Legro RS, Modi BP, Strauss JR. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26:118–124. doi: 10.1016/j.tem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewens KG, Jones MR, Ankener W, Stewart DR, Urbanek M, Dunaif A, et al. FTO and MC4R gene variants are associated with obesity in polycystic ovary syndrome. PLoS One. 2011;6:e16390. doi: 10.1371/journal.pone.0016390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista MC, Duarte EF, Borba MD, Zingler E, Mangussi-Gomes J, Dos SB, et al. Trp28Arg/Ile35Thr LHB gene variants are associated with elevated testosterone levels in women with polycystic ovary syndrome. Gene. 2014;550:68–73. doi: 10.1016/j.gene.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JY, Lee EJ, Jin GM, Sung YA, Lee HJ, Heon KS, et al. Genome-wide association study identifies GYS2 as a novel genetic factor for polycystic ovary syndrome through obesity-related condition. J Hum Genet. 2012;57:660–664. doi: 10.1038/jhg.2012.92. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44:1020–1025. doi: 10.1038/ng.2384. [DOI] [PubMed] [Google Scholar]
- 11.Mayer SB, Evans WS, Nestler JE. Polycystic ovary syndrome and insulin: our understanding in the past, present and future. Womens Health (Lond Engl) 2015;11:137–149. doi: 10.2217/whe.14.73. [DOI] [PubMed] [Google Scholar]
- 12.Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:66–71. doi: 10.1210/jc.2004-0229. [DOI] [PubMed] [Google Scholar]
- 13.Lerchbaum E, Schwetz V, Giuliani A, Obermayer-Pietsch B. Influence of a positive family history of both type 2 diabetes and PCOS on metabolic and endocrine parameters in a large cohort of PCOS women. Eur J Endocrinol. 2014;170:727–739. doi: 10.1530/EJE-13-1035. [DOI] [PubMed] [Google Scholar]
- 14.Kulshreshtha B, Singh S, Arora A. Family background of diabetes mellitus, obesity and hypertension affects the phenotype and first symptom of patients with PCOS. Gynecol Endocrinol. 2013;29:1040–1044. doi: 10.3109/09513590.2013.829446. [DOI] [PubMed] [Google Scholar]
- 15.Dewailly D, Hieronimus S, Mirakian P, Hugues JN. Polycystic ovary syndrome (PCOS) Ann Endocrinol (Paris) 2010;71:8–13. doi: 10.1016/j.ando.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. [DOI] [PubMed]
- 17.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 18.Perna GP, Battistoni I, Angelini L. Heart rate modulation in stable ischemic heart disease: what we have learned from the SIGNIFY study? G Ital Cardiol (Rome) 2015;16:155–160. doi: 10.1714/1820.19824. [DOI] [PubMed] [Google Scholar]
- 19.Cwiklinska A, Strzelecki A, Kortas-Stempak B, Zdrojewski Z, Wroblewska M. ApoE-containing HDL and the development of atherosclerosis. Postepy Hig Med Dosw (Online) 2015;69:1–9. doi: 10.5604/17322693.1134724. [DOI] [PubMed] [Google Scholar]
- 20.Eryilmaz OG, Sarikaya E, Gulerman C, Akar S, Cicek N. Endometrial thickness measurement throughout a menstrual cycle in non-obese infertile patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2012;286:1597–1600. doi: 10.1007/s00404-012-2488-y. [DOI] [PubMed] [Google Scholar]
- 21.Christ JP, Vanden BH, Brooks ED, Pierson RA, Chizen DR, Lujan ME. Ultrasound features of polycystic ovaries relate to degree of reproductive and metabolic disturbance in polycystic ovary syndrome. Fertil Steril. 2015;103:787–794. doi: 10.1016/j.fertnstert.2014.12.094. [DOI] [PubMed] [Google Scholar]
- 22.Shah B, Parnell L, Milla S, Kessler M, David R. Endometrial thickness, uterine, and ovarian ultrasonographic features in adolescents with polycystic ovarian syndrome. J Pediatr Adolesc Gynecol. 2010;23:146–152. doi: 10.1016/j.jpag.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 24.Joe-kechebelu NN, Mbamara SU, Ikechebelu JI. Familial trend in polycystic ovarian syndrome: report of two cases. Ann Afr Med. 2013;12:182–184. doi: 10.4103/1596-3519.117630. [DOI] [PubMed] [Google Scholar]
- 25.Moini A, Eslami B. Familial associations between polycystic ovarian syndrome and common diseases. J Assist Reprod Genet. 2009;26:123–127. doi: 10.1007/s10815-009-9297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2006;103:7030–7035. doi: 10.1073/pnas.0602025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Yu G, Yang D, Li S, Lu S, Wu X, et al. Prevalence and predictors of metabolic abnormalities in Chinese women with PCOS: a cross- sectional study. BMC Endocr Disord. 2014;14:76. doi: 10.1186/1472-6823-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran LJ, Teede HJ, Noakes M, Clifton PM, Norman RJ, Wittert GA. Sex hormone binding globulin, but not testosterone, is associated with the metabolic syndrome in overweight and obese women with polycystic ovary syndrome. J Endocrinol Investig. 2013;36:1004–1010. doi: 10.3275/9023. [DOI] [PubMed] [Google Scholar]
- 29.Veltman-Verhulst SM, van Haeften TW, Eijkemans MJ, de Valk HW, Fauser BC, Goverde AJ. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum Reprod. 2010;25:3123–3128. doi: 10.1093/humrep/deq272. [DOI] [PubMed] [Google Scholar]
- 30.Tzeng CR, Chang YC, Chang YC, Wang CW, Chen CH, Hsu MI. Cluster analysis of cardiovascular and metabolic risk factors in women of reproductive age. Fertil Steril. 2014;101:1404–1410. doi: 10.1016/j.fertnstert.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Wallace IR, McKinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clin Endocrinol. 2013;78:321–329. doi: 10.1111/cen.12086. [DOI] [PubMed] [Google Scholar]
- 32.Winters SJ, Gogineni J, Karegar M, Scoggins C, Wunderlich CA, Baumgartner R, et al. Sex hormone-binding globulin gene expression and insulin resistance. J Clin Endocrinol Metab. 2014;99:E2780–E2788. doi: 10.1210/jc.2014-2640. [DOI] [PubMed] [Google Scholar]
- 33.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 31 kb)
(DOCX 18 kb)
(DOCX 18 kb)