Figure 2.
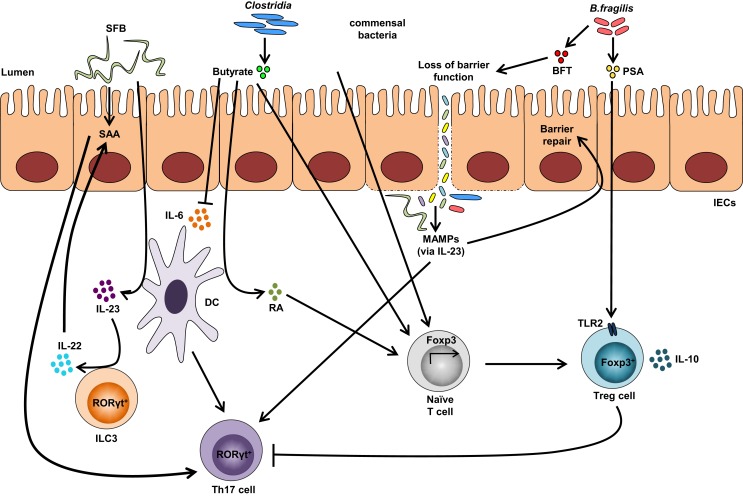
Impact of the gut microbiota on Treg and Th17 immune responses. Colonization with segmented filamentous bacteria (SFB) occurs by intimate attachment to the intestinal epithelium and promotes the development of Th17 cells via intestinal epithelial cell (IEC)-derived cytokines, serum amyloid A (SAA), as well as antigen presentation by dendritic cells (DCs). Adhesion of SFB to IEC can potentially generate a circuit, wherein DC-derived IL-23 stimulates IL-22 production by type 3 innate lymphoid cells (ILC3), which in turn induces SAA from IEC and can lead to Th17 cell differentiation. Conversely, colonization of beneficial commensal bacteria induces de novo generation of Tregs and downregulates Th17 immune responses. Commensal bacteria, including most Clostridia species, produce short-chain fatty acids, i.e., butyrate, which participates in the de novo generation of Tregs by suppressing proinflammatory cytokines, by promoting RA production from DCs, and by inducing Foxp3 transcription. Among different Bacteroides fragilis strains, those expressing polysaccharide A (PSA) mediate the generation of Tregs via TLR2, while those secreting B. fragilis toxin (BFT) alter the function of IEC tight junctions. Upon disruption of barrier function, dissemination of microbial products, recognized by microbe-associated molecular patterns (MAMPs), occurs and activates the IL-23 pathway, resulting in subsequent barrier repair and stimulation of Th17 immune responses.