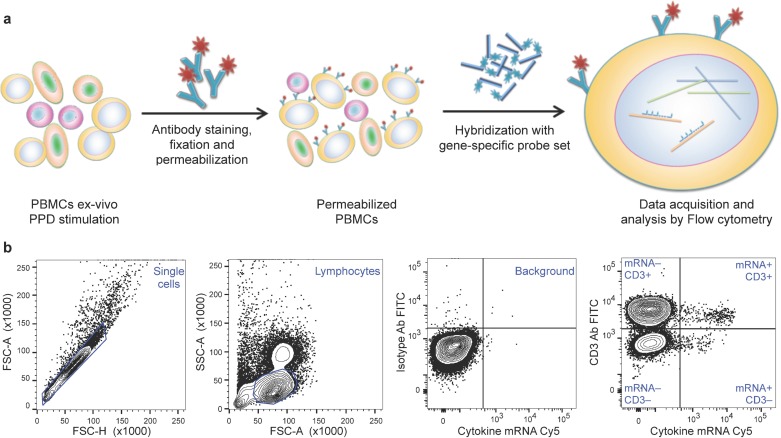
Fig 1. Schematic representation of the dual-color FISH-Flow assay.
(a) Human PBMCs were left unstimulated or stimulated with 10 μg/ml PPD for 6 hr, in the presence of costimulatory monoclonal antibodies (αCD28 and αCD49d, each at 0.1 μg/ml). The cells were then stained with FITC-labeled αCD3 antibody, fixed, permeabilized, and hybridized overnight with Cy5-labeled, gene-specific nucleic acid probes. After hybridization, 100,000 events were acquired on a BD LSRII flow cytometer and analyzed. (b) Gating strategy: Single cells (inside the diagonal gate) were selected in the forward scatter-height (FSC-H) versus forward scatter-area (FSC-A) plot. Lymphocytes were then identified in a FSC-A versus side scatter-area (SSC-A) plot. Unstimulated cells stained with FITC-labeled isotype-control antibody and Cy5-labeled nucleic acid probe were gated in a bivariate plot to identify background. These gates were applied to unstimulated and stimulated cells stained with FITC-labeled αCD3 antibody and Cy5-labeled nucleic acid probe to obtain the frequencies of events in each quadrant. The events in the upper right quadrant (double-positive cells) were used for subsequent analysis.