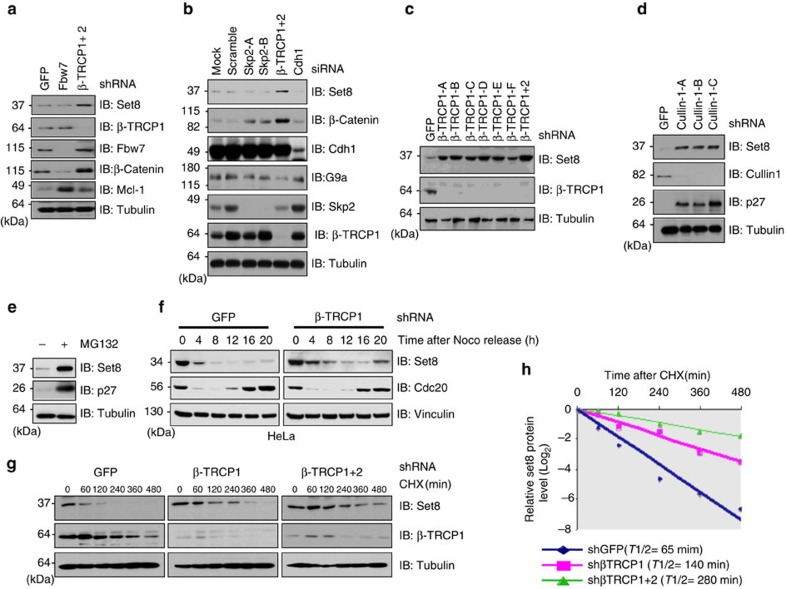
Figure 2. Set8 protein stability is negatively controlled by the SCFβ-TRCP1 E3 ligase complex.
(a) Immunoblot (IB) analysis of whole cell lysates (WCL) derived from HeLa cells infected with the indicated shRNA constructs. (b) IB analysis of HeLa cells transfected with the indicated siRNA oligonucleotides. (c) IB analysis of WCL derived from HeLa cells infected with shRNA constructs specific for GFP, β-TRCP1 (six independent lentiviral β-TRCP1-targeting shRNA constructs namely, -A, -B, -C, -D, -E, -F), or β-TRCP1+2, followed by selection with 1 μg ml−1 puromycin for 3 days to eliminate non-infected cells. (d) IB analysis of WCL from 293 T cells infected with shRNA specific for GFP, or several shRNA constructs against Cullin 1 (three independent lentiviral Cullin 1-targeting shRNA constructs namely, -A, -B, -C) followed by selection with 1 μg ml−1 puromycin for 3 days to eliminate the non-infected cells. (e) IB analysis of WCL derived from 293 T cells with or without MG132 treatment. (f) HeLa cells were infected with the shGFP or shβ-TRCP1 followed by selection with 1 μg ml−1 puromycin for 3 days to eliminate non-infected cells. The generated stable cell lines were then treated with nocodazole to arrest at the M phase, and then release back to the cell cycle by washing off nocodazole. At the indicated time points, WCL were prepared and immunoblots were probed with the indicated antibodies. (g) HeLa cells were infected with the shRNA constructs for GFP, β-TRCP1 or β-TRCP1+2 followed by selection with 1 μg ml−1 puromycin for 3 days to eliminate non-infected cells. The generated stable cell lines were then split into 60-mm dishes. After 20 h, cells were treated with 20 μg ml−1 cycloheximide (CHX). At the indicated time points, WCL were prepared and immunoblots were probed with the indicated antibodies. (h) Quantification of the Set8 band intensities in g. Set8 band intensity was normalized to tubulin, and then normalized to the t=0 controls.