Table 3.
Summary of reported Vps34 inhibitors.
| Vps34 inhibitor | Molecular Structure |
IC50 (in vitro) |
IC50 (in vivo) | IC50 for other lipid kinases | IC50 for protein kinases |
Reference | Patent by |
|---|---|---|---|---|---|---|---|
| Wortmannin | 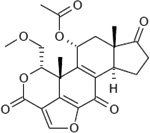 |
1.8 ~ 4.0 nM |
6 µM by counting GFP- 2xFYVE puncta; 30 nM by overall proteolysis in rat hepatocytes |
Wortmannin and its analogues are not inhibitors of Ptdlns-4-kinase (at conc. up to 32 µM). |
Wortmannin and its analogues are not inhibitors of protein kinase C (at conc. up to 22 µM), c-Src tyrosine kinase (at conc. up to 5.5 µM), and phosphoinositide- specific phospholipase C (at conc. up to 0.27 mM). |
[1–4] | |
|
3-methyladenine (3-MA) |
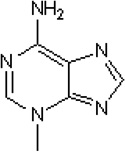 |
~ 1 mM (at 50 µM ATP) |
616 µM by counting GFP- 2xFYVE puncta; 5 mM 3-MA inhibited 60% proteolysis in hepatocytes. |
[1,3,5] | |||
|
LY294002 (2-(4-morpholinyl)-8- phenylchromone) |
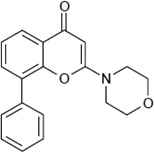 |
1.4 ~ 4.2 µM |
10 µM by overall proteolysis in rat hepatocytes |
[2,3,6] | |||
| PIK-III | 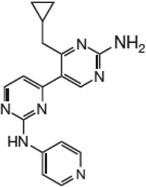 |
18 nM | 55 µM by counting GFP- 2xFYVE puncta; |
>1 µM for PI(3)Kα, PI(3)Kβ, PI(3)Kγ, PI(3)Kδ, PI4Kβ. |
>9.1 µM for mTOR | [1] | Novartis |
|
VPS34-IN1 (1-[{2-[(2- chloropyridin- 4yl)amino]-4’- (cyclopropylmethyl)- [4,5’-bipyrimidin]-2’- yl}amino]-2- methylpropan- 2-ol) (CAS registry number 1383716-33-3) |
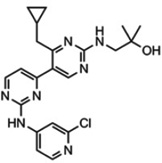 |
25 nM | 100 nM by counting GFP- 2xFYVE puncta; 100 nM ~ 1 µM by SGK3 activity and T- loop/hydrophobic motif phosphorylation |
>1 µM for other lipid kinases in the Dundee panel (19 lipid kinases, includes class I PI(3)Ks), the AstraZeneca panel (8 lipid kinases, includes class I) and ProQuinase panel (13 lipid kinases, includes class I and class II PI(3)Ks); did not significantly inhibit any of the lipid kinases tested including class I (p110α, p110β p110γ and p110δ) and all three members of the class II (PI(3)KC2α, PI(3)KC2β, PI(3)KC2γ) PI(3)Ks. |
1 µM did not significantly inhibit the activity of any of the protein kinases in the Dundee panel (140 kinases) and the ProQinase panel (300 kinases) |
[7] | Novartis (WO 201208 5815 A1) |
|
SAR405 ((8s)-9-[(5- Chloranylpyridin-3- yl)methyl]-2-[(3r)-3- Methylmorpholin-4- yl]-8- (Trifluoromethyl)- 6,7,8,9a-Tetrahydro- 3h-Pyrimido[1,2- A]pyrimidin-4-One) (CAS registry number 1523406-39-4) |
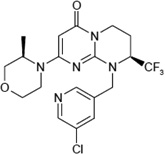 |
1.2 nM (KD=1.5 µM) |
27 nM by counting GFP- 2xFYVE puncta; 419 nM by counting % of cells with GFP- LC3 dots in response to starvation |
In vitro > 10 µM for class I PI(3)Ks (p110α, p110β, p110γ and p110δ) and class II PI(3)Ks (PI(3)KC2α, PI(3)KC2β and PI(3)KC2γ); In vivo: 1 µM leads to 39%, 68%, and 63% inhibition of p110α, p110β and p110δ, respectively. |
In vitro > 10 µM for mTOR; In vivo: 1 µM leads to 52% inhibition of SMG1, but not any other protein kinases. 10 µM did not affect Akt, S6, p53, and phosphorylation. |
[2] | Sanofi |
|
Compound 31 ((2S)-8-[(3R)-3- Methylmorpholin-4- yl]-1-(3-methyl-2- oxobutyl)-2- (trifluoromethyl)-3,4- dihydro-2Hpyrimido[ 1,2-a]pyrimidin-6- one) |
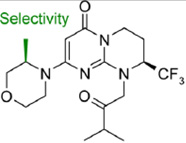 |
2 nM (KD= 2.7 ± 0.9 nM) |
82 nM by counting GFP- 2xFYVE puncta; |
In vitro > 2 µM for class I PI(3)Ks (p110α, p110β, p110γ and p110δ); and > 10 µM for class II PI(3)Ks (PI(3)KC2α and PI(3)KC2γ); |
In vitro > 10 µM for mTOR; In vivo: 8.5 µM for Akt (@Ser473); |
[8] | Sanofi |
References:
. Dowdle, W.E., B. Nyfeler, J. Nagel, R.A. Elling, S. Liu, E. Triantafellow, … L.O. Murphy, Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol, 2014. 16(11): p. 1069–1079.
. Ronan, B., O. Flamand, L. Vescovi, C. Dureuil, L. Durand, F. Fassy, … B. Pasquier, A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol, 2014. 10(12): p. 1013–1019.
Blommaart, E.F., U. Krause, J.P. Schellens, H. Vreeling-Sindelarova, and A.J. Meijer, The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem, 1997. 243(1–2): p. 240–246.
. Powis, G., R. Bonjouklian, M.M. Berggren, A. Gallegos, R. Abraham, C. Ashendel, … et al., Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res, 1994. 54(9): p. 2419–2423.
. Seglen, P.O. and P.B. Gordon, 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A, 1982. 79(6): p. 1889–1892.
. Vlahos, C.J., W.F. Matter, K.Y. Hui, and R.F. Brown, A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem, 1994. 269(7): p. 5241–5248.
. Bago, R., N. Malik, M.J. Munson, A.R. Prescott, P. Davies, E. Sommer, … D.R. Alessi, Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem J, 2014. 463(3): p. 413–427.
. Pasquier, B., Y. El-Ahmad, B. Filoche-Romme, C. Dureuil, F. Fassy, P.Y. Abecassis, … B. Ronan, Discovery of (2S)-8-[(3R)-3-Methylmorpholin-4-yl]-1-(3-methyl-2-oxobutyl)-2-(trifluoromethyl)-3,4-dihydro-2H-pyrimido[1,2-a]pyrimidin-6-one: A Novel Potent and Selective Inhibitor of Vps34 for the Treatment of Solid Tumors. J Med Chem, 2015. 58(1): p. 376–400.
