Figure 9.
Model of the Role of MICU in Arabidopsis.
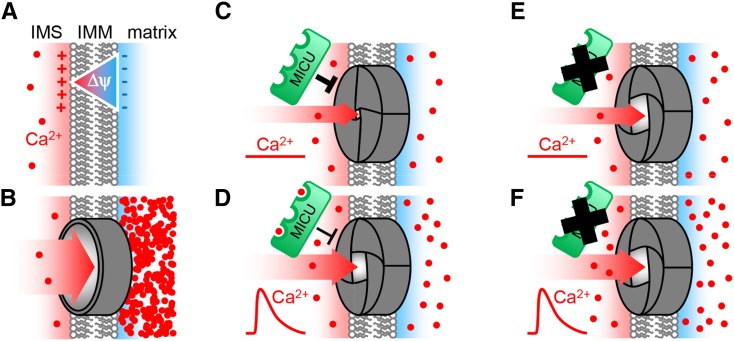
(A) and (B) The impact of a hypothetical mitochondrial inner membrane that is nonpermeable (A) or fully permeable (B) to Ca2+ ions. Lack of permeability would prevent Ca2+ presence, while full permeability would cause Nernstian accumulation in the matrix driven by the steep electrical gradient of the mitochondrial membrane potential ΔΨ. Control of matrix Ca2+ requires an intermediate state between those extremes.
(C) A selective Ca2+ channel, likely to be composed of MCU proteins, provides restricted passage. MICU is localized in the IMS and acts as a throttle for Ca2+ flux by inhibiting the channel. Under steady state conditions, this inhibition allows relatively low Ca2+ levels to be maintained in the matrix, despite the coexistence of the channel and the large membrane potential ΔΨ.
(D) and (E) The presence of three EF-hands and modification of protein conformation by Ca2+ binding provides a mechanistic basis for the inhibitory effect of MICU to be tuned during Ca2+ transients (D). In the absence of MICU, its inhibitory effect on the channel is lifted, resulting in elevated steady state levels of free Ca2+ in the matrix based on an increased influx rate (E).
(F) When Ca2+ levels rise in the cytosol and IMS, e.g., by a physiological stimulus, uptake into the matrix can occur at an increased rate and free Ca2+ accumulates at higher levels. The remaining control is likely to be provided by buffering and export systems and the biophysical properties as well as abundance of the channel itself. Additional channel regulators may also contribute, but no homologs of mammalian candidates exist in Arabidopsis. Our data support this model both in vitro and in the bona fide in vivo situation of intact Arabidopsis root tissue.