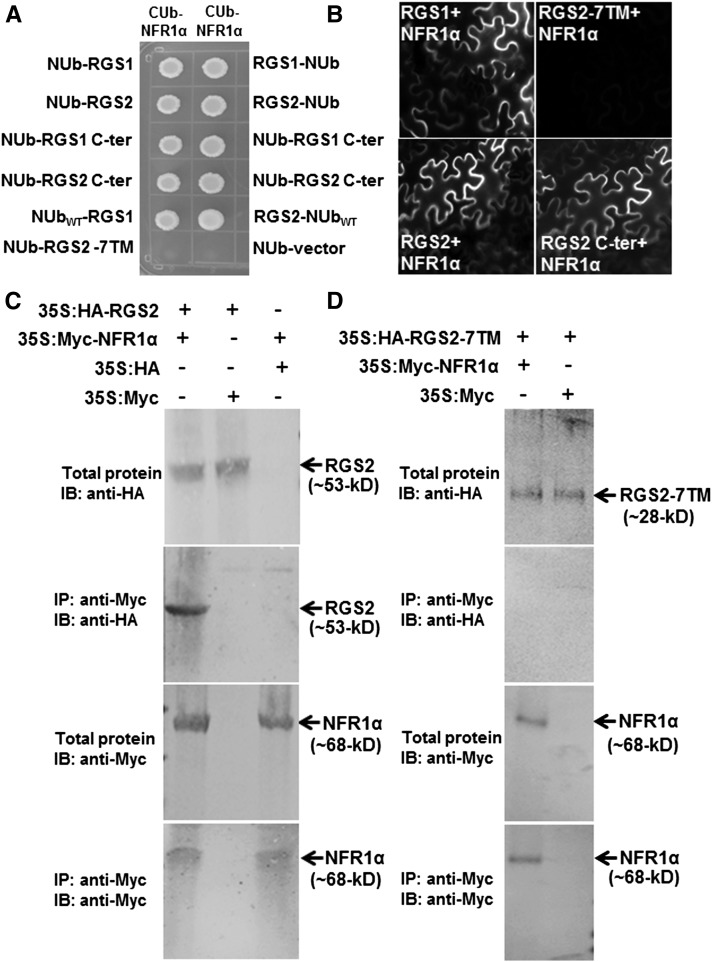
Figure 7.
Soybean RGS Proteins Interact with NFR1 Receptors.
(A) Interaction between RGS and NFR using a split ubiquitin-based interaction assay. The picture shows yeast growth on selective media with 200 µM methionine. In all cases, full-length RGS proteins, the N-terminal seven-transmembrane region (7TM), and the C-terminal RGS domain containing RGS proteins were used as NUb fusions in both orientations (NUb-RGS denoting NUb fused to the N terminus of RGS and RGS-NUb denoting NUb fused to the C terminus of RGS). NFR1α was used for the CUb fusion. NUbwt and NUb-vector fusion constructs were used as positive and negative controls, respectively. Two biological replicates of the experiment were performed with identical results.
(B) Interaction between RGS (in 77-nEYFP-N1) and NFR1 (in 78-cEYFP-N1) proteins using BiFC assay. Agrobacteria containing different combinations of RGS1 and RGS2 and NFR1α were infiltrated in tobacco leaves, and reconstitution of YFP fluorescence due to protein-protein interaction was visualized under a Nikon Eclipse E800 microscope with epifluorescence modules. At least four independent infiltrations were performed for each protein combination with similar results.
(C) Interaction between RGS and NFR1α protein using an in vivo co-IP assay. Anti-Myc antibody can pull down HA-tagged RGS2 from total protein extracts of plants expressing 35S:HA-RGS2 and 35S:Myc-NFR1α (lane 1) but not from total protein extracts from plants expressing 35S:HA-RGS2 and Myc-tagged EV (lane 2) or 35S:Myc-NFR1α and HA-tagged EV (lane 3).
(D) The N-terminal seven-transmembrane domain of RGS2 did not interact with NFR1α in a similar in vivo co-IP assay.