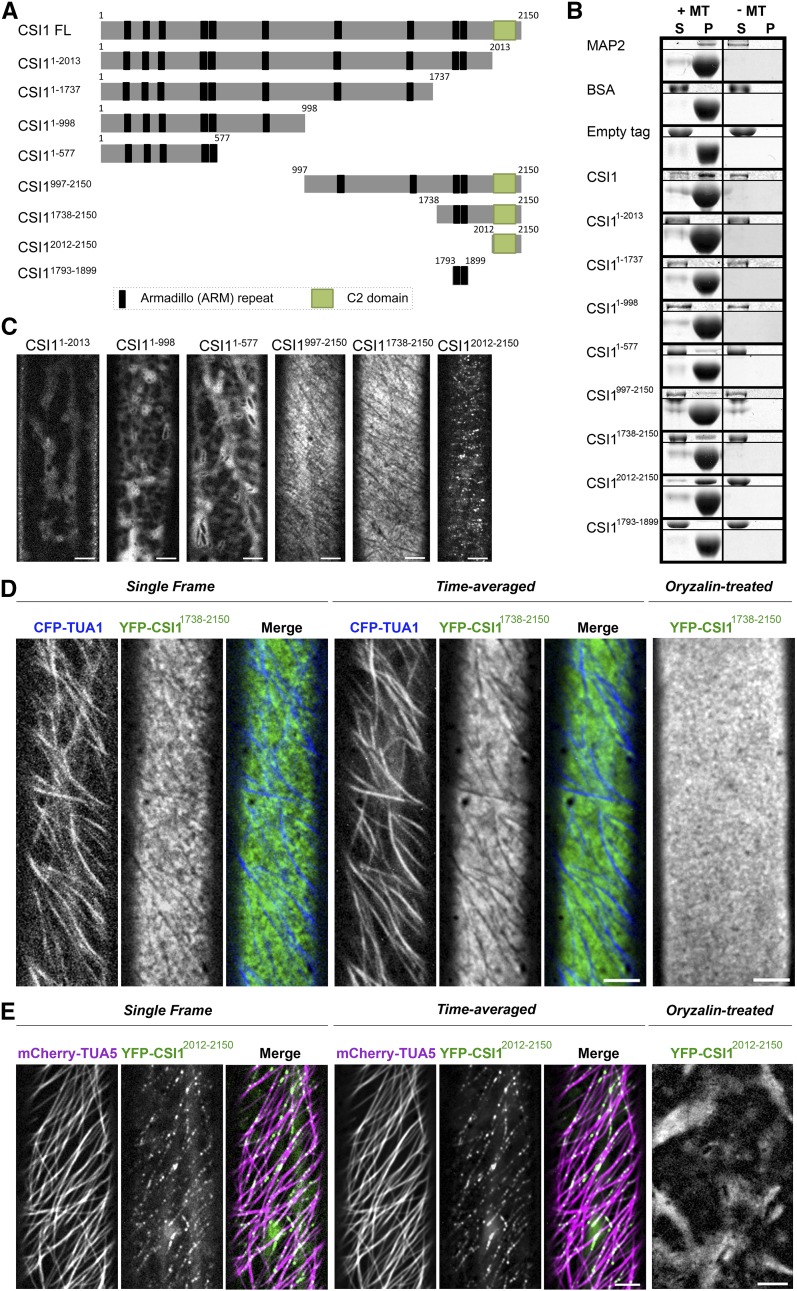
Figure 1.
The C Terminus of CSI1 Is Required for Microtubule Binding in Vitro and Colocalization with Microtubules in Vivo.
(A) Schematic diagram of the CSI1 full-length protein (CSI1 FL) and different truncations. Black bars represent predicted ARM repeats, and green boxes represent the C2 domain.
(B) C2 domain binds to microtubules in vitro. Equal amounts of His-tagged proteins were used in the presence (+) or absence (−) of taxol-stabilized microtubules (MTs). MAP2 was used as a positive control. BSA and empty tag were used as negative controls. For each microtubule binding experiment, representative gels from three technical replicates are shown. Upper panel shows purified proteins, and lower panel shows tubulin. S, supernatant; P, pellet.
(C) YFP-tagged CSI1 truncations are localized to different compartments in vivo. Arabidopsis seedlings expressing YFP-tagged CSI1 truncations were grown in the dark for 3 d before imaging. Single optical sections in epidermal cells ∼2 mm below the apical hook were imaged by confocal microscopy. Bars = 5 μm.
(D) YFP-CSI11738-2150 localizes at PM excluding underlying microtubules. Arabidopsis seedlings coexpressing YFP-CSI11738-2150 and CFP-TUA1 were imaged by confocal microscopy. Single optical sections, time average of 61 frames (2-min duration; 2-s interval), and merged images are shown. YFP-CSI11738-2150 lost its distinct localization after 5 h treatment with 20 μM oryzalin. Bars = 5 μm.
(E) YFP-CSI12012-2150 colocalizes with cortical microtubules. Single optical sections, time average of 61 frames (2-min duration; 2-s interval), and merged images of seedlings coexpressing YFP-CSI12012-2150 and mCherry-TUA5 are shown. YFP-CSI12012-2150 lost its distinct localization after 5 h of treatment with 20 μM oryzalin. Bars = 5 μm.