Figure 7. Regulation of Swi6 dynamics and the role of Swi6 in heterochromatin spreading, boundary formation, and repression.
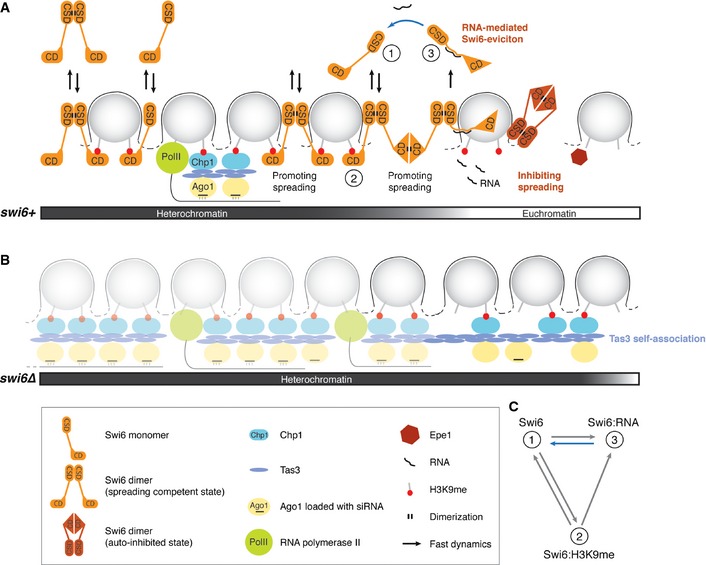
- Model for the formation of a distinct heterochromatic domain. The spreading‐competent open conformation of Swi6 dimers is fully dispensable or acts redundantly with RNAi‐mediated spreading mechanisms in the formation of constitutive heterochromatin. The spreading of heterochromatin into neighboring euchromatin in the absence of DNA‐encoded boundary elements, such as TFIIIC binding sites, can be stopped by the spreading‐incompetent closed conformation of Swi6 dimers, RNA‐mediated eviction of spreading‐competent Swi6, or Epe1 activity. Swi6 molecules interact with H3K9me2 only transiently and exchange between free and RNA‐bound forms (2, 1, or 3, respectively; see also C). Removal of RNA from Swi6 constitutes a rate‐limiting step in the Swi6 exchange cycle (blue arrow) and contributes to tight repression of heterochromatin.
- In the absence of Swi6, H3K9 methylation is mediated by the RITS complex (Ago1, Chp1, Tas3) and can spread beyond natural boundaries via self‐associating Tas3. This might occur independently of siRNAs.
- In theory, Swi6 can exist in at least three kinetically distinct populations: 1) freely diffusible Swi6, 2) Swi6 bound to H3K9me2, and 3) Swi6 bound to RNA; 3 is the least and 1 the most mobile population. The blue arrow indicates the rate‐limiting step in the Swi6 exchange cycle. Under physiological conditions, most Swi6 molecules exchange between the RNA‐ and H3K9me2‐bound forms.
