Figure 1. Molecular view of complement activation, amplification, and regulation.
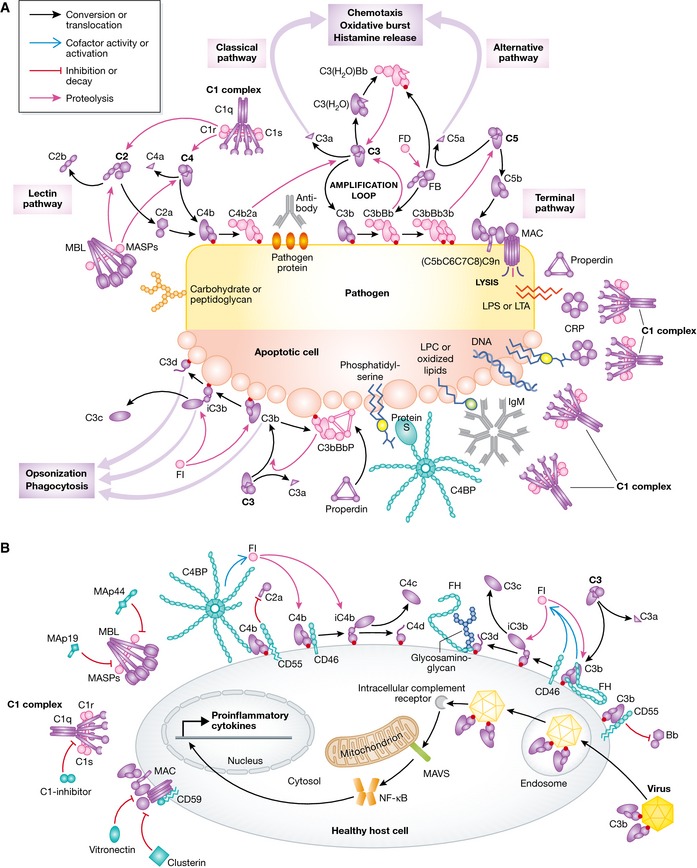
(A) Pattern recognition molecules sense the presence of pathogens and altered self. In the classical pathway, C1q (within the C1 complex) recognizes PAMPs (pathogen‐specific proteins, lipopolysaccharide (LPS), lipoteichoic acid (LTA), peptidoglycan) or DAMPs (DNA, phosphatidylserine, oxidized lipids, lysophosphatidylcholine (LPC)) either directly or antibody‐bound. This recognition induces autoactivation of C1r, which subsequently activates C1s. This is followed by cleavage of C4 and C2 by C1s and the subsequent formation of the CP C3 convertase C4b2a. Cleavage of C4 exposes an internal thioester, which causes C4b to become covalently attached to the activator surface, in turn tethering the convertase activity to the activator. In the lectin pathway, patterns of glycans are detected via MBL, CL‐LK, or ficolins leading to activation of MASPs and formation of the same C3 convertase, C4b2a. C3 convertases cleave C3 into C3b, which also becomes covalently attached to the activator surface. Surface‐associated C3b recruits FB, which leads to FB activation and the formation of C3bBb, the AP C3 convertase, which cleaves more C3 and amplifies complement activation. In addition to the surface‐bound C3 convertase, a fluid‐phase convertase can be formed by association of water‐reacted C3, termed C3(H20), to FB thus constantly maintaining a low level of complement activation in solution (tick‐over). Both of the surface‐bound C3 convertases can bind a C3b molecule whereby the C5 convertases are formed. These cleave C5 into C5a and C5b, thus initiating the terminal pathway and leading to formation of the membrane attack complex (MAC). Complement opsonins and PRMs are shown in purple, whereas the proteolytically active complexes are shown in light pink. (B) Complement activation and amplification are attenuated on host surfaces. The healthy cells express membrane‐bound or attract soluble regulators that irreversibly dissociate convertases (DAF, CR1, FH, C4BP) and act as cofactors for FI‐mediated degradation of C3b and C4b (MCP, FH, CR1, C4BP) or prevent MAC assembly (CD59). Soluble regulators also prevent formation of the MAC (clusterin, vitronectin). Recently, it was discovered that complement mediates a potent intracellular immune response to non‐enveloped viruses. Deposition and covalent attachment of C3 onto pathogens in the extracellular environment serve as a marker of cellular invasion because C3 products in the cytosol are detected by an as yet unidentified receptor. This receptor signals through MAVS and induces an antiviral state by triggering the transcription of pro‐inflammatory cytokines. Intracellular complement immunity is independent of professional immune cells and is conserved in mammals.
