Figure 3. Complement factor H family regulators.
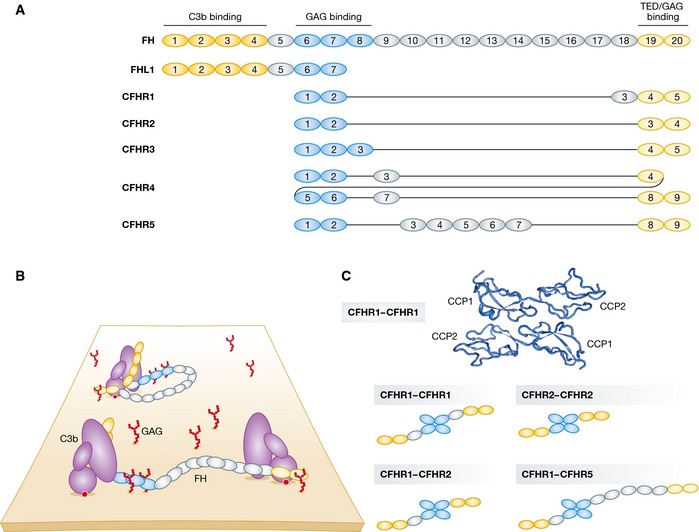
(A) Domain organization of complement factor H (FH). C3‐binding domains are highlighted in yellow and glycosaminoglycan (GAG)‐contacting domains in blue. Complement factor H‐like 1 (FHL1) and complement factor H‐related (CFHR) proteins are represented below according to their sequence similarity to FH. CFHRs share high sequence similarity with each other and with FH. All CFHRs contain domains homologous to the FH GAG‐ and TED‐binding domains but lack the domains homologous to the FH N‐terminal CCP1–4. (B) Models of FH recruited to non‐activating surfaces. FH may be recruited to the self‐surface‐bound C3b and establish bivalent contacts with one C3b molecule. Owing to its flexible structure, FH may bind two surface‐bound C3b molecules (or iC3b and C3d). (C) CFHRs form homo‐ and heterodimers. The N‐terminal CCP1–2 domains of CFHR1 crystallize as head‐to‐tail dimers (RSCB ID 3ZD2). Due to the high sequence identity between the CCP1–2 of CFHR1, 2, and 5, the three proteins are able to form homo‐ and heterodimers in the serum and thus modulate complement activation in a more complex manner.
