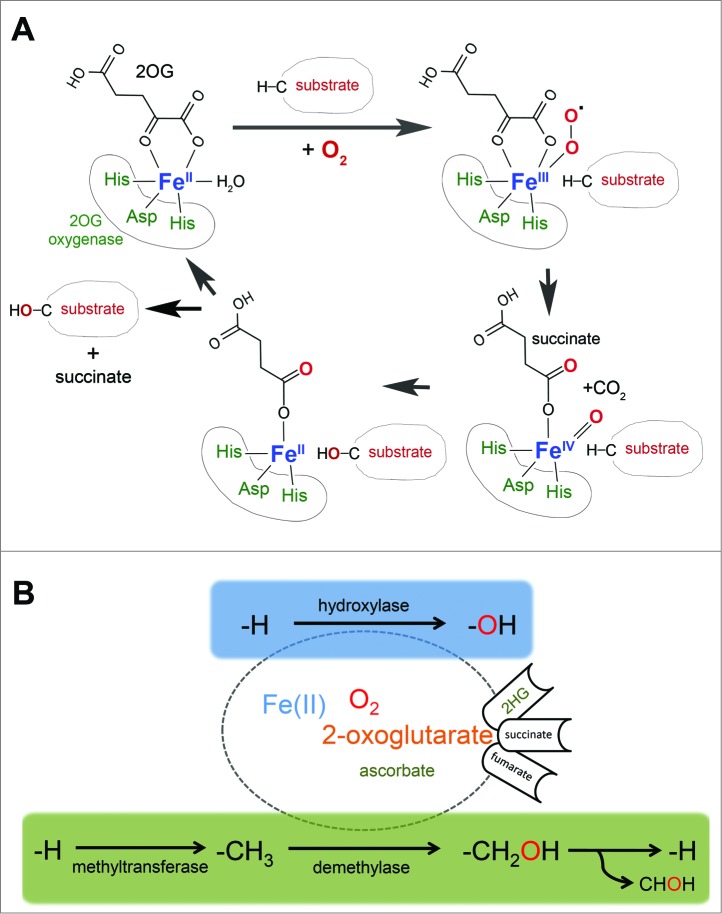
Figure 1.
2-oxoglutarate-oxygenase catalysis.(A) Catalytic cycle. Catalysis requires essential co-factors Fe(II), molecular oxygen (O2), and the Krebs cycle intermediate 2-oxoglutarate (2OG), together with the ‘2-His 1-carboxylate’ motif (His-Asp-His) within the active site of the enzyme. Note that one atom of oxygen from molecular oxygen is incorporated into the product, and that the reaction generates succinate and carbon dioxide. (B) 2OG-oxygenases catalyze stable hydroxylation (blue box) and demethylation via hydroxylation (green box) of DNA, RNA, lipid and protein. Note that ascorbate is required for full activity of a subset of 2OG-oxygenases (hence the smaller font). Hydroxylation of a methyl group generally creates a highly unstable hydroxymethyl intermediate that decomposes into formaldehyde (CHOH) and the unmodified residue. The fate of the formaldehyde is not known, but may be metabolized by formaldehyde dehydrogenase. Note that in some chemical contexts hydroxylation of a methyl group can create a stable hydroxymethyl product, such as that catalyzed by TETs. The oncometabolite 2-hydroxyglutarate (2HG) can interfere with 2OG-oxygenase function by acting as an activating co-substrate in some instances, or as a 2OG competitive inhibitor in others. Succinate and fumarate inhibit 2OG-oxygenases by product inhibition and 2OG competition, respectively.