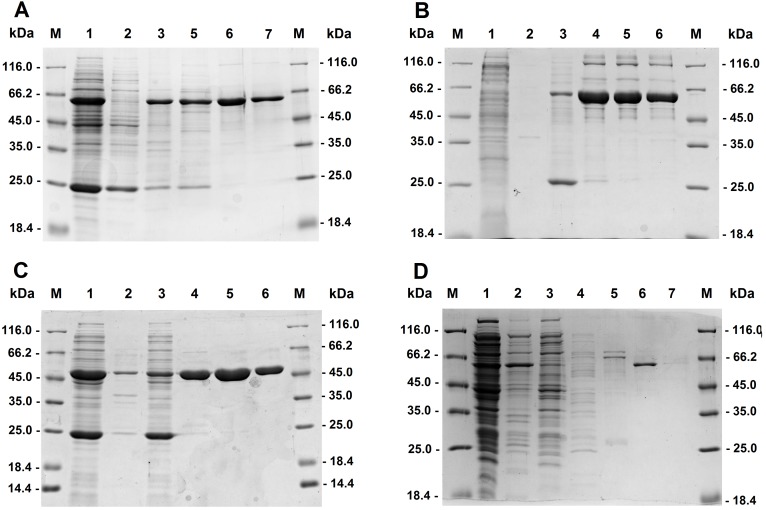
Fig 3. SDS-PAGE (12.5%) of fractions obtained during purification of recombinant fumarases FumA (A), FumB (B) and FumC (C) from E. coli K-12 as well as of a recombinant mesaconase/fumarase FumD from E. coli O157:H7 (ATCC 700728) (D).
M, molecular mass standard proteins; lane 1, cell extract of E. coli producing the corresponding proteins; lane 2, membrane fraction; lane 3, flow through after Ni-NTA column; lane 4, elution with 25 mM imidazole; lane 5, elution with 50 mM imidazole; lane 6, elution with 300 mM imidazole; lane 7 with 500 mM imidazole. The fractions eluted with 300 mM imidazole were used for the following enzyme characterization. Proteins were stained with Coomassie blue. The predicted molecular mass of native FumA is 60.3 kDa, that of FumB 60.1 kDa, that of FumC 50.5 kDa, and that of FumD 60.1 kDa.