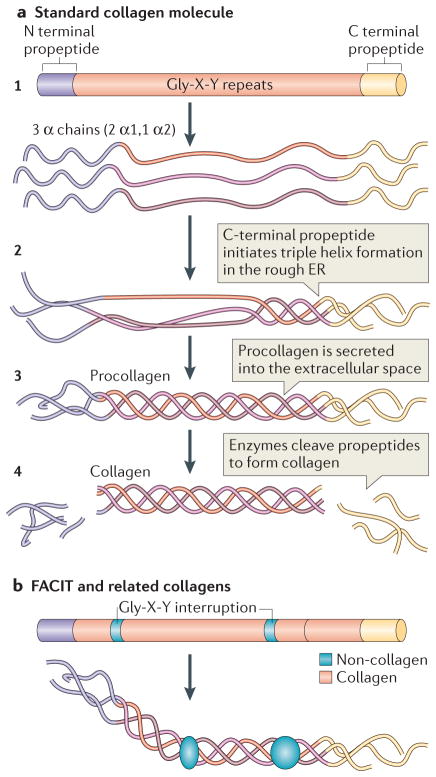
Figure 1. Collagen structure.
a | The standard fibrillar collagen molecule is characterized by amino- and carboxy-terminal propeptide sequences, which flank a series of Gly-X-Y repeats (where X and Y represent any amino acids but are frequently proline and hydroxyproline) (step 1). These form the central triple helical structure of procollagen and collagen. Three α-chains (the illustration shows two α1-chains and one α2-chain, which is representative of type I collagen) are intracellularly assembled into the triple helix following initiation of this process by the C-terminal domain (step 2). Procollagen is secreted by cells into the extracellular space (step 3) and converted into collagen by the removal of the N- and C-propeptides via metalloproteinase enzymes (step 4).
b | Fibril-associated collagens with interrupted triple helices (FACIT) and related collagens have a different structure to standard fibrillar collagen; they contain non-collagenous regions — that is, non-triple helical sequences. These lead to kinks in the resulting macromolecular structure that straighten under small strains. Figure part a is modified, with permission, from REF 135 © 2012 Fan et al.; licensee BioMed Central Ltd, and from REF. 136, Klug, William S.; Cummings, Michael R., Concepts of Genetics, 5th Edition, © 1997. Reprinted by permission of Pearson Education, Inc., Upper Saddle River, NJ. Figure part b: this figure was originally published in Biochem. J. Jäälinoja, J., Ylöstalo, J., Beckett, W., Hulmes, D. J. S. & Ala-Kokko, L., Trimerization of collagen IX alpha-chains does not require the presence of the COL1 and NC1 domains. Biochem. J. 2008; 409: 545–554 © the Biochemical Society.