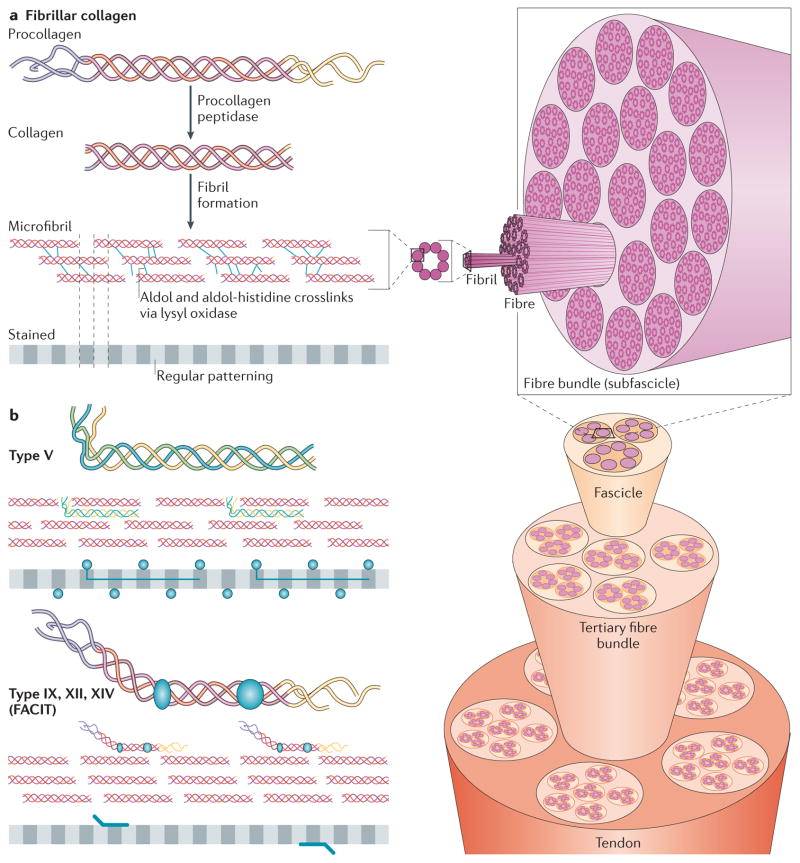
Figure 2. Fibrillar collagen assembly.
a | After procollagen is secreted into the extracellular space, collagen type-specific metalloproteinase enzymes remove the amino- and carboxy-propeptides. Collagen is assembled into cross-striated microfibrils that occur in the extracellular matrix of connective tissues. This can be observed via electron microscopy as regularly spaced bands, as represented by the grey, striped bar. The position of non-fibrillar collagens in this structure is shown in similar diagrams in part b. Short microfibrils merge into mature fibrils through longitudinal and axial growth. To form mature fibres, lysyl oxidase catalyses the formation of intramolecular and intermolecular covalent crosslinks between collagen molecules. Fibres are bundled together within a connective tissue and stabilized via interactions with fibril-associated collagens with interrupted triple helices (FACIT) collagens and small leucine-rich repeat proteoglycans (SLRPs) into linear structures capable of transmitting tensile forces. Specifically in the case of tendons, fibres ranging in size from 1 μm to 20 μm are bundled into 15 400 μm diameter triangular subfascicles, subfascicles are bundled into 150 1000 μm fascicles and fascicles are grouped into tertiary bundles ranging from 1000 μm to 3000 μm. Finally, multiple tertiary bundles are enclosed by the epitenon connective tissue, which contains the vascular, lymphatic and nerve sources for the tendon tissue.
b | In addition to fibrillar collagen, other collagen subtypes, such as type V and FACIT collagens, are incorporated into the fibril structure. Type V collagen is inserted between strands of the microfibril, and FACIT collagens cling to the surface of the microfibril and work with SLRPs to stabilize higher order structures. Figure part a is modified, with permission, from REF. 135 © 2012 Fan et al.; licensee BioMed Central Ltd, from Klug, William S.; Cummings, Michael R., Concepts of Genetics, 5th Edition, © 1997 (REF. 136). Reprinted by permission of Pearson Education, Inc., Upper Saddle River, NJ, and from REF. 138, reprinted from Trends Biotechnol., 26, Liu, Y., Ramanath, H. S. & Wang, D.-A., Tendon tissue engineering using scaffold enhancing strategies, 201–209, Copyright (2008), with permission from Elsevier. Figure part b: this figure was originally published in Biochem. J. Jäälinoja, J., Ylöstalo, J., Beckett, W., Hulmes, D. J. S. & Ala-Kokko, L., Trimerization of collagen IX alpha-chains does not require the presence of the COL1 and NC1 domains. Biochem. J. 2008; 409: 545–554 © the Biochemical Society.