Abstract
Human rhinovirus (RV) is a common cause of acute respiratory infection (ARI) in children. We aimed to characterize the clinical and demographic features associated with different RV species detected in children attending hospital with ARI, from low‐income families in North‐east Brazil. Nasopharyngeal aspirates were collected from 630 children <5 years with ARI. Clinical diagnosis and disease severity were also recorded. Samples were analyzed by multiplex PCR for 18 viral and atypical bacterial pathogens; RV positive samples underwent partial sequencing to determine species and type. RV was the fourth commonest pathogen accounting for 18.7% of pathogens detected. RV was commonly detected in children with bronchiolitis, pneumonia, and asthma/episodic viral wheeze (EVW). Species and type were assigned in 112 cases (73% RV‐A; 27% RV‐C; 0% RV‐B). Generally, there were no differences in clinical or demographic characteristics between those infected with RV‐A and RV‐C. However, in children with asthma/EVW, RV‐C was detected relatively more frequently than RV‐A (23% vs. 5%; P = 0.04). Our findings highlight RV as a potentially important pathogen in this setting. Generally, clinical and demographic features were similar in children in whom RV‐A and C species were detected. However, RV‐C was more frequently found in children with asthma/EVW than RV‐A. J. Med. Virol. 88:58–63, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: rhinovirus, child, respiratory tract infections, bronchiolitis, pneumonia, asthma
INTRODUCTION
Human rhinoviruses (RV) are a major cause of upper respiratory tract infection and acute wheezy episodes in pre‐school children and asthmatics [Simons et al., 2005; Renwick et al., 2007; Arden and Mackay, 2010; Gern, 2010]. Traditionally, they have been divided into two species (A & B) and over 100 serotypes. In 2006, a number of novel RV strains were identified with significant variation in the viral protein (VP) regions (VP2, VP4) and 5′ non‐coding region (5′ NCR); these formed a distinct new species that was designated “rhinovirus C” (RV‐C) [Arden et al., 2006; Lamson et al., 2006; Arden and Mackay, 2010]. Approximately 60 RV‐C types are now recognized based on phylogenetic clustering, and these viruses have been associated with severe acute respiratory infection (ARI), particularly wheezing ARIs, in young children [Lau et al., 2007; Dominguez et al., 2008; Bizzintino et al., 2011] and asthmatics [Lemanske et al., 2005; Miller et al., 2009; Calvo et al., 2010].
Although the role of RV‐C infection as a cause of ARI in children is increasingly recognized in the developed world, its disease‐causing role in low‐income settings is relatively unknown. In this study, we sought to characterize the clinical and demographic features associated with different RV species detected in children attending hospital with ARI from low‐income families in North‐east Brazil.
METHODS
Setting and Study Design
This study was undertaken as part of a larger research project investigating the viral and atypical bacterial causes of paediatric ARI in a low income setting in Brazil [Bezerra et al., 2011; Fawkner‐Corbett et al., 2012]. This prospective cross‐sectional study was conducted between April 2008 and March 2010 at the Instituto de Medicina Integral Prof Fernando Figueira (IMIP), a large publically funded teaching hospital in Recife, Pernambuco, North‐east Brazil. Between one third and one half of the population of Recife lives in relative poverty in urban slums and favelas with IMIP's paediatric emergency department (ED) primarily providing primary, secondary, and tertiary medical care to these low‐income families.
Subjects
All children aged less than 5 years with upper and/or lower respiratory tract manifestations of ARI of less than 7 days duration were eligible for inclusion. A trained research assistant approached consecutive patients with ARI between 7 am and 3 pm (Monday to Friday) whilst they were in the paediatric ED and collected clinical and demographic data from parents/guardians. Baseline observations including temperature, oxygen saturation, respiratory, and pulse rates were recorded at the time of arrival in the ED.
One research assistant collected all nasopharyngeal aspirate (NPA) samples within 4 hr of the child seeing a doctor in the ED using a standardized protocol [Semple et al., 2005].
Ethics Statement
The Ethics Committee at IMIP and the National Research Ethics Office of Brazil approved the study and written informed consent was obtained from parents/guardians prior to enrolment.
Clinical Manifestations of ARI
The clinical outcome and diagnosis for each child were recorded following discharge from the hospital. The discharge diagnosis for each child was made by attending physicians not involved in the study and based on standard clinical criteria. Thus bronchiolitis was diagnosed in children <18 months in whom upper respiratory symptoms preceded lower respiratory symptoms of wheeze, tachypnoea and signs of respiratory distress. Pneumonia was diagnosed in children with fever, tachypnoea, and respiratory distress where focal or diffuse crackles or decreased vesicular sounds were present on auscultation. Most diagnoses of pneumonia were based on clinical criteria alone, but radiographic findings were used in some. A diagnosis of asthma/episodic viral wheeze (EVW) was made in children in whom discreet episodes of wheeze occurred, often in association with a presumed viral upper respiratory tract infection. Upper respiratory tract infections (including croup) were diagnosed based on symptoms such as coryza, earache, sore throat, and stridor.
Pathogen Detection
Respiratory samples were stored at −70°C and transported to Liverpool, UK where multiplex real‐time PCR (RT‐PCR) was undertaken as previously described for 18 viral and atypical bacterial pathogens including human rhinovirus (RV), respiratory syncytial virus (RSV), human metapneumovirus (hMpV), influenza (flu), parainfluenza virus (PIV) 1–4, bocavirus (hBoV), coronavirus (CoV), adenovirus (AdV), Mycoplasma pneumonia (Myc), and Chlamydophila pneumonia (Cpp) [Bezerra et al., 2011; Fawkner‐Corbett et al., 2012].
RNA samples from children in whom RV had been identified by RT‐PCR were reverse transcribed to cDNA. Random primers, AMV‐RT, RNAse inhibitor and dNTPs (Promega, Southampton, Hampshire, UK) were added to each sample at 4°C. The RNA samples were reverse transcribed using a thermocycler TC‐512 machine (Techne, Stone, Staffordshire, UK) with conditions of 35 min at 42°C, 20 min at 50°C, and finally 5 min at 85°C. All cDNA was then stored at −30°C prior to transportation to Perth, Australia for further analysis [Lee et al., 2007].
Analysis of Rhinovirus Strain
In Perth, cDNA was amplified using semi‐nested PCR primers specific to the 390 bp variable region in the 5′ non‐coding region (5′ NCR) of the RV genome, employing primers [Bochkov et al., 2014] and cycling conditions described previously [Bizzintino et al., 2011; Lee et al., 2007,2007] The Australian Genome Research Facility sequenced the PCR products. RV genotypes were then assigned based on comparisons of the 5′ NCR sequences with those of the 101 classical serotypes as well as the 52 newly identified genotypes by phylogenetic tree analysis using the ClustalX software (Conway Institute, University College Dublin, Dublin, Ireland). Representative samples of each genotype have previously been sequenced at the VP4–VP2 coding region to confirm the species assignment [Lee et al., 2012].
Samples that could not be successfully typed in Perth were sent to Wisconsin for cloning, sequencing, and species assignment as described previously [Lee et al., 2007,2007].
Statistical Analysis
Statistical analysis was performed using SPSS v18.0.0 (SPSS Inc, Chicago, IL). Differences in hospital admissions, clinical presentations and co‐infection rates were calculated using the χ2 test. Variation between groups was assessed with Kruskal–Wallis test. Differences in age were calculated using the Mann–Whitney U test with a P‐value <0.05 considered significant.
RESULTS
Clinical and Demographic Features of Children With RV Infection
Of 630 children presenting with ARI, initial RT‐PCR in Liverpool detected RV in 118 (18.7%). Analysis in Perth/Wisconsin confirmed the RV detection and identified strains in 112 (17.8%). In the 6/118 samples in which RV detection was not confirmed, coxsackievirus was identified in three samples, and poliovirus (1/HEV‐C, strain Sabin 1), enterovirus, and no virus at all in the remainder. RV was the fourth most prevalent pathogen detected after RSV (37%), Adenovirus (25%), and Bocavirus (19%) [Fawkner‐Corbett et al., 2012].
Of the 112 children (55% male) in whom RV detection was confirmed, the median (range) age was 6 (0–48) months, with 95/112 (85%) being under 18 months of age and 85/112 (76%), 12 months or less. In 62/112 (55%) patients, the RV‐associated ARI necessitated hospital admission. Twenty‐one children (19%) had a history of low birth weight and 46/112 (41%) had a member of the immediate household who smoked. Bronchiolitis (67/112; 60%) was the most prevalent ARI diagnosis, followed by pneumonia (21%), asthma/EVW (10%), and URTI (5%). Overall no significant clinical or demographical differences were found between children in whom RV was and was not detected (Table I).
Table I.
Demographic Information for Children With RV‐A, RV‐C, and Non‐RV ARI. In the Non‐RV Group, Viruses/Atypical Bacteria Were Detected in 440/518 Children
| RV‐A | RV‐C | NON‐RV ARI | |
|---|---|---|---|
| N | 82 | 30 | 518 |
| Gender | 46 (56%) Male | 16 (53%) Male | 283 (55%) Male |
| Median age (range) | 6.5 Months (1–48) | 6.0 Months (0–48) | 7.0 Months (0–57) |
| Birth weight <2,500G | 16 (20%) | 5 (17%) | 71 (14%) |
| Breast fed | 73 (89%) | 28 (93%) | 479 (92%) |
| Co‐morbidities | 4 (5%) | 0 (0%) | 28 (5%) |
| Smokers at home | 37 (45%) | 9 (30%) | 217 (42%) |
| Hospital admission | 49 (60%) | 13 (43%) | 277 (54%) |
| Co‐detection | 49 (60%) | 16 (53%) | 205 (39%) |
Co‐detection of other viruses and atypical bacteria was found in 65/112 (58%) RV‐positive samples, with the commonest viruses co‐detected being AdV (29/65; 45%), hBoV (39%), and RSV (20%). Children in whom RV was detected were significantly more likely to have another pathogen detected (RV+ve, 58%; RV−ve, 39%; χ2 = 13.5 P < 0.001). Viral or atypical bacterial co‐detection was not associated with particular clinical presentations or severities of disease (data not shown).
Clinical and Demographic Features of Children With RV‐C Infection
Of 112 RV samples, RV‐A was detected in 82/112 (73%) and RV‐C detected in 30/112 (27%); no RV‐B was detected. In three samples, two RV‐A strains were detected (Supplementary Table S1). These were samples from a 3 month old male (types A20 and A11), an 8 month old male (A47, A44), and an 11 month old male (A78, A47). We identified a novel strain of RV‐A (A106), in an 8 month male with multiple co‐infections (AdV, PIV, and hBoV), diagnosed with bronchiolitis who required hospital admission for 6 days. There was no significant difference in clinical or demographic data between those infected with RV‐A or RV‐C (Table I). Levels of co‐infection were similar in patients in whom RV‐A and RV‐C were detected (60% vs. 53%).
Clinical Presentations
RV was commonly detected in children with bronchiolitis, pneumonia and asthma/EVW (Fig. 1). In children with asthma/EVW, RV‐C was detected relatively more frequently than RV‐A. Thus, 7/30 (23%) children with RV‐C infection presented with asthma/EVW, whereas only 4/82 (5%) children with RV‐A infection presented in this way (χ2 8.44 P = 0.04). In children with pneumonia, RV‐A was more frequently detected than RV‐C (RV‐A, 26%; RV‐C, 10%), but this did not reach statistical significance (P = 0.059).
Figure 1.
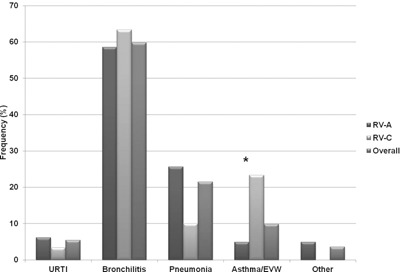
Relative RV detection frequency in different clinical presentations of ARI in pre‐school children (URTI, upper respiratory tract infection; Asthma/EVW, asthma/episodic viral wheeze; * = Significant difference, P < 0.05).
Seasonality
The monthly frequency with which RV‐A and C strains were detected over the study period is shown in Figure 2. Generally, both strains were found to circulate during most months apart from February–April following the dry season. There was a peak in presentation following the rainy season (July and November).
Figure 2.
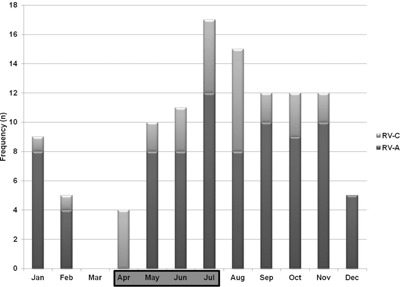
Frequency of RV infections each month (April–July shaded to indicate the local “rainy” season).
DISCUSSION
Using genetic amplification techniques, we successfully classified 112 RV samples from pre‐school children from predominantly low‐income families, presenting with ARI to a children's hospital in Recife, Brazil. Most RV clinical presentations in this cohort attending hospital were in children less than 1 year with lower respiratory tract symptoms, particularly bronchiolitis, pneumonia, and asthma/EVW. Overall the prevalence of RV‐A was three‐times that of RV‐C, but in children with asthma/EVW, RV‐C was detected relatively more frequently than RV‐A.
RV accounted for 17.8% of children presenting to hospital with ARI over the 2 year period, the fourth most prevalent pathogen in the study. This is similar to other epidemiological studies that report the prevalence of RV of 12–26% in ARI [Jartti et al., 2004; Miller et al., 2007; Wang et al., 2010]. In our cohort, RV‐C was detected in 4.7% of ARI cases over the 2 years. Another study in Brazil (São Paulo) in 120 children <12 years presenting as outpatients with ARI detected RV in 47% and RV‐C in 28% of children [Moreira et al., 2011]. Our study differed in both age groups included (<5 years [most <1year], vs. <12 years) and setting (ED vs. outpatient). Our cohort was also from a predominantly low income background [Bezerra et al., 2011]. Our finding of 27% of RV samples being RV‐C positive is comparable to similar studies in children in Spain and China that report a prevalence of 35% and 36%, respectively [Jin et al., 2009; Calvo et al., 2010]. Although we were surprised not to detect RV‐B at all in our cohort, it is often only found at very low levels (0–3%) or in relatively asymptomatic patients [Loens et al., 2006; Lau et al., 2007; Dominguez et al., 2008].
This study supports others suggesting an association between RV‐C and severe ARI, particularly wheezing ARI, in young children. Recent in vitro studies have shown that, in contrast to other RV species, RV‐C grows optimally at either 34°C or 37°C, potentially allowing it to infect the lower respiratory tract as easily as the upper respiratory tract [Ashraf et al., 2013]. It is likely that other factors are also involved, however, given that we found similar rates of infection with RV‐C and A in infants with bronchiolitis and a trend towards more RV‐A than RV‐C in children with pneumonia.
The strengths of this study are that it is one of the largest on RV epidemiology [Onyango et al., 2012], with good quality clinical and demographic data, and samples, collected systematically by one research assistant, from children with both RV‐A and C infection. It supports other studies [Mansbach et al., 2008] suggesting that RV might be an important cause of bronchiolitis [Bezerra et al., 2011]. This is also the first study to examine RV seasonality in Brazil. Unlike previous studies in Australia and North America, RV infection appears to occur throughout the year with peaks during the rainy season, a pattern also seen in Trinidad [Matthew et al., 2009]. It may be that the high population density within our cohort results in continual RV transmission throughout the year.
However, despite recruiting a relatively large cohort of children with RV, the number of children with RV‐C was only 30 and no children had RV‐B. Our cohort was also from one center only and so may not have been representative of other hospitals within Brazil or further afield. We can also not provide an estimate of the precise prevalence of RV infection in this population as data on numbers of children presenting to IMIP with ARI over this time period were not available.
In conclusion we have shown that RV does cause significant lower respiratory disease in young pre‐school children and that in our cohort, RV‐C was more frequently associated with presentations of asthma or episodic viral wheeze than RV‐A.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Table S1: Distribution of strains from samples of RV‐A and RV‐C species (All shown as frequency (n)).
ACKNOWLEDGMENT
Rose K., Fonceca A.M., Hopkins M., Britto M., Cuevas L.E., Correia J.B.
REFERENCES
- Arden KE, Mackay IM. 2010. Newly identified human rhinoviruses: Molecular methods heat up the cold viruses. Rev Med Virol 20:156–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. 2006. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 78:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S, Brockman‐Schneider R, Bochkov YA, Pasic TR, Gern JE. 2013. Biological characteristics and propagation of human rhinovirus‐C in differentiated sinus epithelial cells. Virology 436:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra PGM, Britto MCA, Correia JB, Duarte Mdo C, Fonceca AM, Rose K, Hopkins MJ, Cuevas LE, McNamara PS. 2011. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS ONE 6:18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC, McMinn PC, Goldblatt J, Gern JE, Le Souëf PN. 2011. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 37:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. 2014. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol 52:2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo C, Casas I, Garcia‐Garcia ML, Pozo F, Reyes N, Cruz N, García‐Cuenllas L, Pérez‐Breña P. 2010. Role of rhinovirus C respiratory infections in sick and healthy children in Spain. Pediatr Infect Dis J 29:717–720. [DOI] [PubMed] [Google Scholar]
- Dominguez SR, Briese T, Palacios G, Hui J, Villari J, Kapoor V, Tokarz R, Glodé MP, Anderson MS, Robinson CC, Holmes KV, Lipkin WI. 2008. Multiplex MassTag‐PCR for respiratory pathogens in pediatric nasopharyngeal washes negative by conventional diagnostic testing shows a high prevalence of viruses belonging to a newly recognized rhinovirus clade. J Clin Virol 43:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawkner‐Corbett DWMM, doCarmo MMBD, Rose KMM, Fonceca AP, Britto MMD, Bezerra PMD, Hopkins M, Britto M, Cuevas LE, Correia JB, McNamara PS. 2012. The impact of the H1N1 influenza pandemic on clinical presentations. Pediatr Infect Dis J 31:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gern JE. 2010. The ABCs of rhinoviruses, wheezing, and asthma. J Virol 84:7418–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jartti T, Lehtinen P, Vuorinen T, Osterback R, van den Hoogen B, Osterhaus AD, Ruuskanen O. 2004. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis 10:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Yuan X‐H, Xie Z‐P, Gao H‐C, Song J‐R, Zhang R‐F, Xu ZQ, Zheng LS, Hou YD, Duan ZJ. 2009. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J Clin Microbiol 47:2895–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, Dean A, St George K, Briese T, Lipkin WI. 2006. MassTag polymerase‐chain‐reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza‐like illness in New York state during 2004–2005. J Infect Dis 194:2004–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SKP, Yip CCY, Tsoi HW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV‐C, associated with acute respiratory illness in children. J Clin Microbiol 45:3655–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W‐M, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, et al. 2007. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE 2:966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W‐M, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Jakiela B, Lemanske RF Jr, Shult PA, Gern JE. 2007. High‐throughput, sensitive, and accurate multiplex PCR‐microsphere flow cytometry system for large‐scale comprehensive detection of respiratory viruses. J Clin Microbiol 45:2626–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W‐M, Lemanske RF, Evans MD, Vang F, Pappas T, Gangnon R, Jackson DJ, Gern JE. 2012. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med 186:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanske RF, Jr. , Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, Carlson‐Dakes KT, Adler KJ, Gilbertson‐White S, Pappas TE, Dasilva DF, Tisler CJ, Gern JE. 2005. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 116:571–577. [DOI] [PubMed] [Google Scholar]
- Loens K, Goossens H, de Laat C, Foolen H, Oudshoorn P, Pattyn S, Sillekens P, Ieven M. 2006. Detection of rhinoviruses by tissue culture and two independent amplification techniques, nucleic acid sequence‐based amplification and reverse transcription‐PCR, in children with acute respiratory infections during a winter season. J Clin Microbiol 44:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach JM, McAdam AJ, Clark S, Hain PD, Flood RG, Acholonu U, Camargo CA Jr. 2008. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med 15:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew J, Pinto Pereira, Swenson T, Grindle C, Roberg K, Lemanske RF, Lee WM, Gern JE. 2009. Distribution and seasonality of rhinovirus and other respiratory viruses in a cross‐section of asthmatic children in Trinidad, West Indies. Italian J Pediatr 35:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, Iwane MK, Edwards KM; New Vaccine Surveillance Network. 2007. Rhinovirus associated hospitalizations in young children. J Infect Dis 195:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, Lu X, Williams JV; New Vaccine Surveillance Network. 2009. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 123:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LP, Kamikawa J, Watanabe AS, Carraro E, Leal E, Arruda E, Granato CF, Bellei NC. 2011. Frequency of human rhinovirus species in outpatient children with acute respiratory infections at primary care level in Brazil. Pediatr Infect Dis J 30:612–614. [DOI] [PubMed] [Google Scholar]
- Onyango CO, Welch SR, Munywoki PK, Agoti CN, Bett A, Ngama M, Myers R, Cane PA, Nokes DJ. 2012. Molecular epidemiology of human rhinovirus infections in Kilifi, coastal Kenya. J Med Virol 84:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick N, Schweiger B, Kapoor V, Liu Z, Villari J, Bullmann R, Miething R, Briese T, Lipkin WI. 2007. A recently identified rhinovirus genotype is associated with severe respiratory‐tract infection in children in Germany. J Infect Dis 196:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, Shears P, Smyth RL, Hart CA. 2005. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis 191:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons E, Schroth MK, Gern JE. 2005. Analysis of tracheal secretions for rhinovirus during natural colds. Pediatr Allergy Immunol 16:276–278. [DOI] [PubMed] [Google Scholar]
- Wang W, Cavailler P, Ren P, Zhang J, Dong W, Yan H, Mardy S, Cailhol J, Buchy P, Sheng J, Fontanet A, Deubel V. 2010. Molecular monitoring of causative viruses in child acute respiratory infection in endemo‐epidemic situations in Shanghai. J Clin Virol 49:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Table S1: Distribution of strains from samples of RV‐A and RV‐C species (All shown as frequency (n)).


