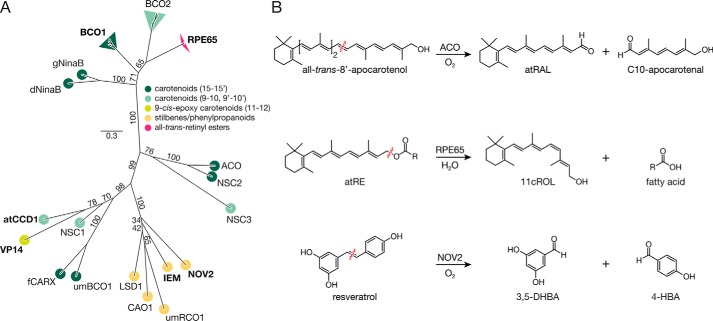
FIGURE 1.
Phylogenic and enzymatic relationships among CCOs. A, unrooted maximum likelihood phylogenic tree of select CCO family members. The tree termini are colored according the substrate specificity of the associated taxon/clade. The BCO1, BCO2, and RPE65 clades are composed of sequences from Mus musculus, Rattus norvegicus, Homo sapiens, and Bos taurus. The altitudes of the triangles associated with these clades are proportional to the evolutionary diversity among the examined taxa. Bootstrap values from 1000 pseudo-replicates are displayed as percentages beside the associated branches. The scale bar indicates the average number of site substitutions over the indicated distance. Members of the CCO family whose reaction mechanisms have previously been examined by isotope labeling experiments are shown in bold. Abbreviations are as follows: dNinaB, Drosophila melanogaster NinaB; gNinaB. Galleria mellonella NinaB; ACO. Synechocystis sp. PCC 6803 ACO; NSC. Nostoc sp. PCC 7120 carotenoid oxygenase; NOV2, Novosphingobium aromaticivorans CCO 2; IEM, Pseudomonas nitroreducens isoeugenol monooxygenase; umRCO1, Ustilago maydis resveratrol cleavage oxygenase 1; CAO1, Neurospora crassa carotenoid oxygenase 1; LSD1, Pseudomonas paucimobilis lignostilbene dioxygenase 1; umBCO1, U. maydis β-carotene cleavage oxygenase 1; fCARX, Fusarium fujikuroi carotenoid oxygenase; VP14, Zea mays viviparous 14; atCCD1, Arabidopsis thaliana carotenoid cleavage dioxygenase 1. B, reactions catalyzed by CCOs considered in this study. Synechocystis ACO, a prototypical CCO member, specifically cleaves all-trans-8′-apocarotenol at the 15,15′-double bond position to generate all-trans-retinal (RAL) and 8′-hydroxy-15′-apocarotenal (C10-apocarotenal). RPE65, an atypical CCO member, catalyzes a coupled ester cleavage/isomerization of all-trans-retinyl esters (atRE) rather than oxidative carotenoid cleavage. This reaction produces 11-cis-retinol (11cROL), a key intermediate in the regeneration of visual chromophore required for vertebrate vision. NOV2 from Novosphingobium cleaves resveratrol at the interphenyl double bond to produce 3,5-DHBA and 4-HBA. Wavy red lines indicate the location of the scissile bond in each reaction.