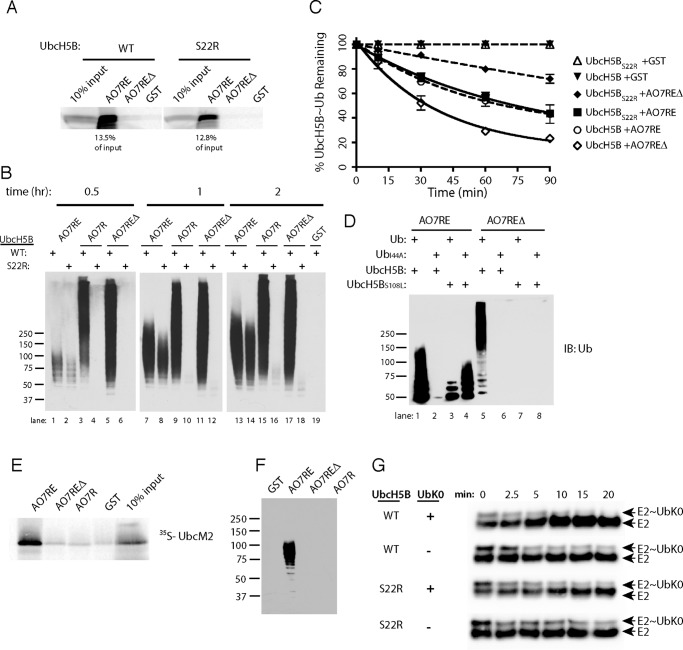
FIGURE 5.
Role of backside binding in AO7 ubiquitination. A, binding assays were carried out as in Fig. 1B, percent of input bound is indicated. B, time course of ubiquitination was carried out as in Fig. 4B. C, loss of E2∼Ub was assessed as in Fig. 4E. Data are from three independent experiments. D, ubiquitination was carried out as in Fig. 4B. E, binding assay was carried out as in Fig. 1B. IB, immunoblot. F, ubiquitination assay was carried out as in Fig. 4B for 90 min using UbcM2 as the E2. G, purified, bacterially expressed WT or S22R UbcH5B was loaded with equimolar amounts of ubiquitin lacking Lys (UbK0), and the release of UbK0 was assessed during incubation in the presence or absence of added UbK0 (160 μm) and with AO7REΔ from which GST had been cleaved. UbcH5B was detected by immunoblotting. Note the ratio of E2∼Ub to E2 over the time course. Efficiency of loading is less than in experiments using 35S-labeled E2 and WT ubiquitin (e.g. Fig. 4F), as the amount of UbK0 used was kept low during loading to maximize the effect of UbK0 added during the discharge phase.