FIGURE 2.
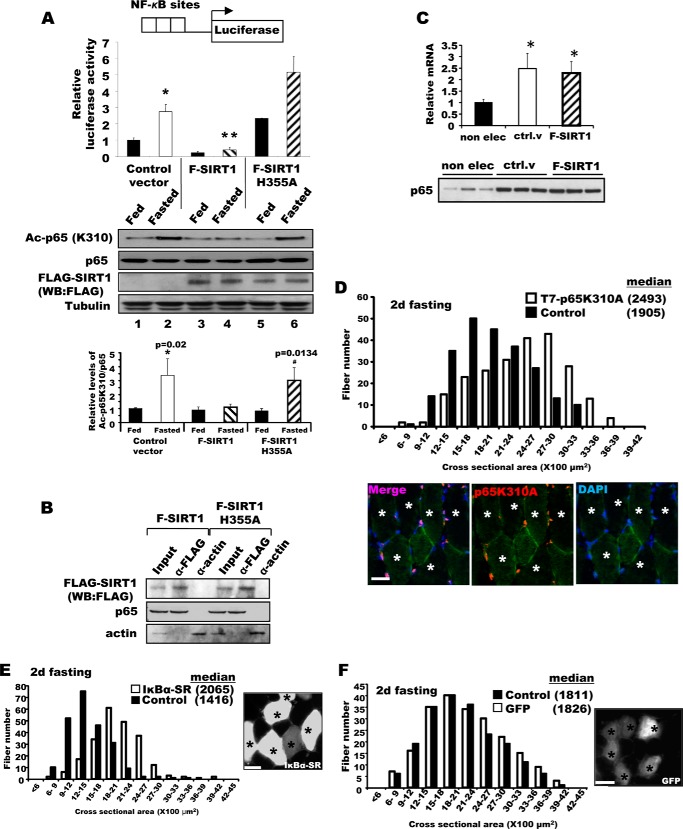
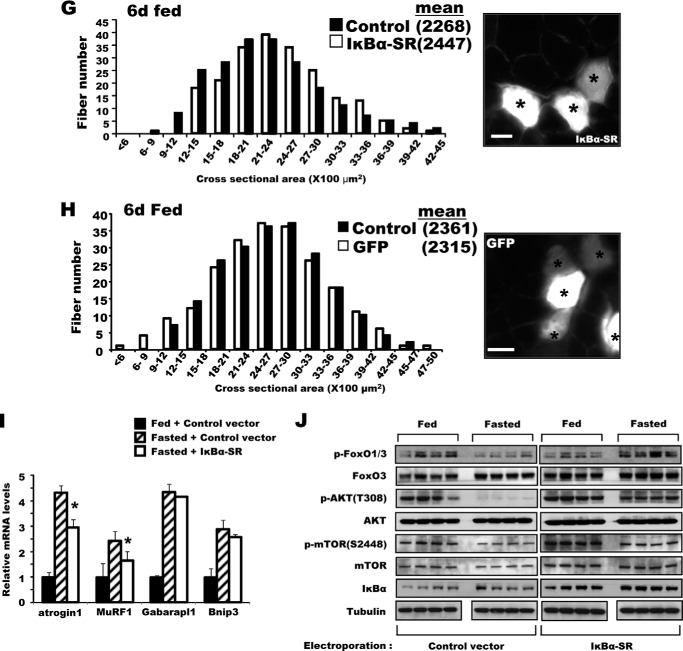
Inhibition of NF-κB activity in adult muscle-reduced muscle wasting upon fasting. A, the increase in NF-κB activity in muscles upon fasting was blocked by SIRT1 overexpression, but was enhanced by overexpression of the inactive mutant SIRT1H355A. NF-κB reporter constructs were co-electroporated into TA muscles with F-SIRT1, F-SIRT1H355A, or a control vector. After food deprivation for 2 days, luciferase activity was measured. n = 3; *, p < 0.05 versus Fed; **, p < 0.05 versus Control vector in Fasted. Lower panel, fasting induced acetylation of the p65 subunit of NF-κB, especially after electroporation of the H355A mutant, but WT SIRT1 decreased acetylation of p65 (but not total p65). B, SIRT1 associates with p65 in fasted TA muscle. Constructs for F-SIRT1 or F-SIRT1H355A genes were electroporated, and 2 days later, muscle lysates were prepared for an immunoprecipitation assay with an antibody against FLAG. After extensive washing with a buffer containing 500 mm KCl, proteins bound to FLAG antibodies were eluted with FLAG peptides and subjected to SDS-PAGE for Western blot (WB). α-Actin was used as a nonspecific control protein. Input corresponds to 10% of the lysates used for immunoprecipitation. One-fourth of the eluate was used for FLAG-SIRT1 and one-half for p65. C, increase in p65 mRNA level upon electroporation. TA muscles of fed mice that are not electroporated (non elec) or electroporated with a control vector (ctrl. v) or FLAG-SIRT1 (F-SIRT1) were used to measure the levels of p65 mRNA (upper) and protein (lower, 10 μg of lysate per lane) (A). n = 4; *, p < 0.05 versus non electroporated muscle. D, the constructs encoding the T7-p65K310A mutant were electroporated into TA muscles. Four days later, mice were fed or deprived of food for 2 days. Fibers expressing p65K310A were stained with a T7 antibody (marked by asterisks), and cross-sectional areas were measured. Scale bar, 20 μm. E, IκBα-SR overexpression protected muscle fibers from atrophy upon fasting. Bicistronic DNA constructs encoding IκBα-SR and GFP were electroporated into TA muscles, and mice were deprived of food for 2 days and cross-sectional areas were measured. Typical image showing the electroporated fibers (GFP-labeled and marked by asterisks) by fluorescence microscopy (right image) is shown. Scale bar, 15 μm. F, GFP overexpression (marked by asterisks) does not influence the size of muscle fibers in either fasted animals. Electroporation of TA muscle with DNA constructs encoding GFP, food deprivation procedures, and measurement of fiber sizes were performed as described in the legend to Fig. 2B. Scale bar, 20 μm. G, IκBα-SR electroporation does not induce any increase in muscle size in fed mice. Six days after electroporation of IκBα-SR, cross-sectional areas were measured. Scale bar, 20 μm. H, GFP overexpression (marked by asterisks) does not alter fiber sizes in fed mice. Scale bar, 20 μm. I, overexpression of IκBα-SR inhibits the expression in fasting of atrogin1 and MuRF1 but not autophagy genes. TA muscles were electroporated with constructs for either a control vector or IκBα-SR. Four days later, mice were fed or deprived of food for 2 days. n = 3; *, p < 0.05 versus Fasted + Control vector. J, after IκBα-SR overexpression, the levels of phosphorylated AKT and FoxO3 in muscles of fasted mice. TA muscles were electroporated with IκBα-SR or a control vector, and the animals were fed or deprived of food as in D. Muscle extracts were analyzed for different components of AKT-FoxO pathway by Western blot.