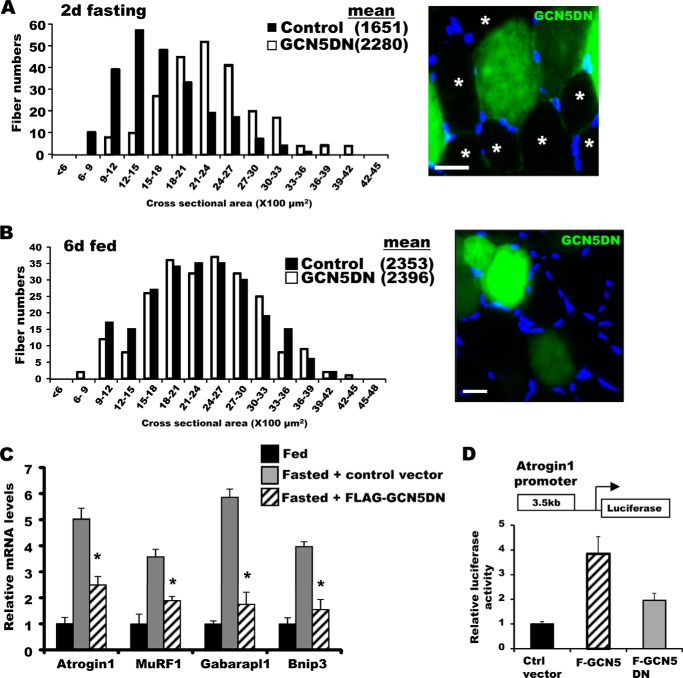
FIGURE 4.
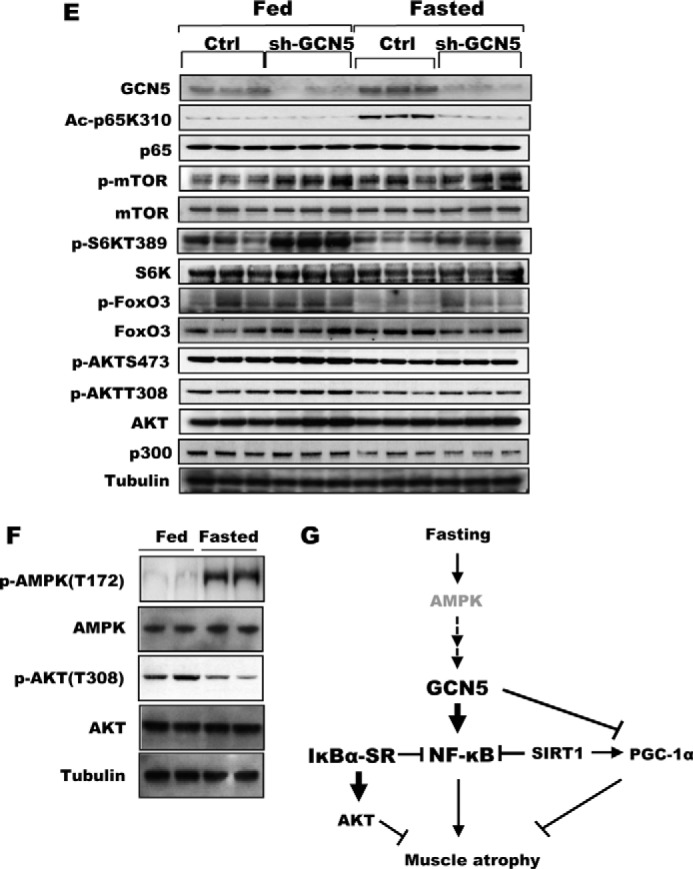
Inhibition of GCN5 by a dominant-negative mutant attenuated wasting and sustained mTOR/S6K activity. A, bicistronic plasmids encoding a GCN5 mutant that lacks acetyltransferase activity and GFP were electroporated into TA muscles. Four days later, mice were deprived of food for 2 days, and cross-sectional areas were measured. A representative image showing the electroporated (GFP) and non-electroporated fibers (marked by asterisks) (right image). Scale bar, 20 μm. B, GCN5 inhibition by overexpression of its dominant-negative mutant (GCN5DN) does not alter size of muscle fibers in fed mice. Frequency histograms show the distribution of the cross-sectional areas of muscle fibers. Scale bar, 20 μm. C, inhibition of GCN5 activity repressed induction of atrogenes upon fasting. TA muscles were electroporated with constructs for either a control vector or FLAG-GCN5DN. Four days later, mice were fed or deprived of food for 2 days. n = 3; *, p < 0.05 versus Fasted + Control vector. D, transient transfection of GCN5 into C2C12 cells elevated the activity of the atrogin1 promoter. C2C12 myoblasts were transiently transfected with plasmids encoding a reporter gene together with FLAG-GCN5, FLAG-GCN5DN, or a control vector, and cell lysates were prepared for the reporter assay. n = 3; *, p < 0.05. E, knockdown of GCN5 maintained mTOR/S6K activity at a high level in fasting. TA muscles were electroporated with sh-GCN5, and the animals were deprived of food for 2 days. Muscle extracts were analyzed for several different components of the mTOR/S6K pathway by Western blot. F, AMPK activation in skeletal muscle upon fasting for 2 days. The levels of phospho-AMPK and phospho-AKT were examined with the muscles lysates of fed or fasted mice. G, summary of actions of NF-κB and GCN5 in the muscle identified here. New findings about the regulation of muscle wasting in this study are highlighted in bold.