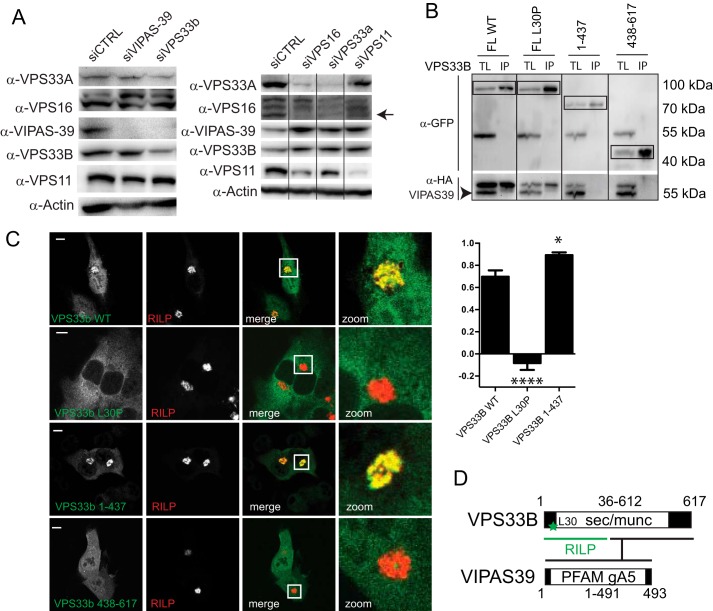
FIGURE 5.
VPS33B and VIPAS39 are not HOPS complex subunits and mutations in VPS33B differentially affect VPS33B interactions with VIPAS39 and RILP. A, lysates of MelJuSo cells silenced for indicated HOPS subunits were analyzed by WB using anti-VPS16, anti-VPS33B, anti-VIPAS39, anti-VPS11, anti-VPS33, and anti-actin (as the loading control) antibodies as indicated. Within each panel, experimental conditions were run on the same blot with the same exposures for each detection antibody, and cutouts were taken and grouped for presentation purposes. B, Immunoprecipitates (IP) with anti-GFP from lysates of MelJuSo cells co-expressing GFP-VPS33B mutants and HA-VIPAS39 (as indicated) were analyzed by WB using anti-GFP and anti-HA antibodies. Experimental conditions were run on the same blot with the same exposures for each detection antibody, and cutouts were taken and grouped for presentation purposes. TL, total lysate. C, MelJuSo cells expressing GFP-VPS33B constructs (green) and mRFP-RILP (red) were fixed and imaged by CLSM. Scale bars: 10 μm. Correlation coefficient was calculated from plot profiles measuring RILP intensity and GFP intensity over a vector through the cells. Graphs show the average correlation coefficient ± S.E (n > 25) between VPS33B constructs and RILP (*, p ≤ 0.05; ****, p ≤ 0.0001, ns = not significant, when compared with VPS33B wt). D, summary of VPS33B domains involved in interactions with RILP (green) and VIPAS39. Asterisks indicate Leu-30 (L30) residue (mutated in ARC syndrome) required for VPS33B recruitment to RILP.