FIGURE 5.
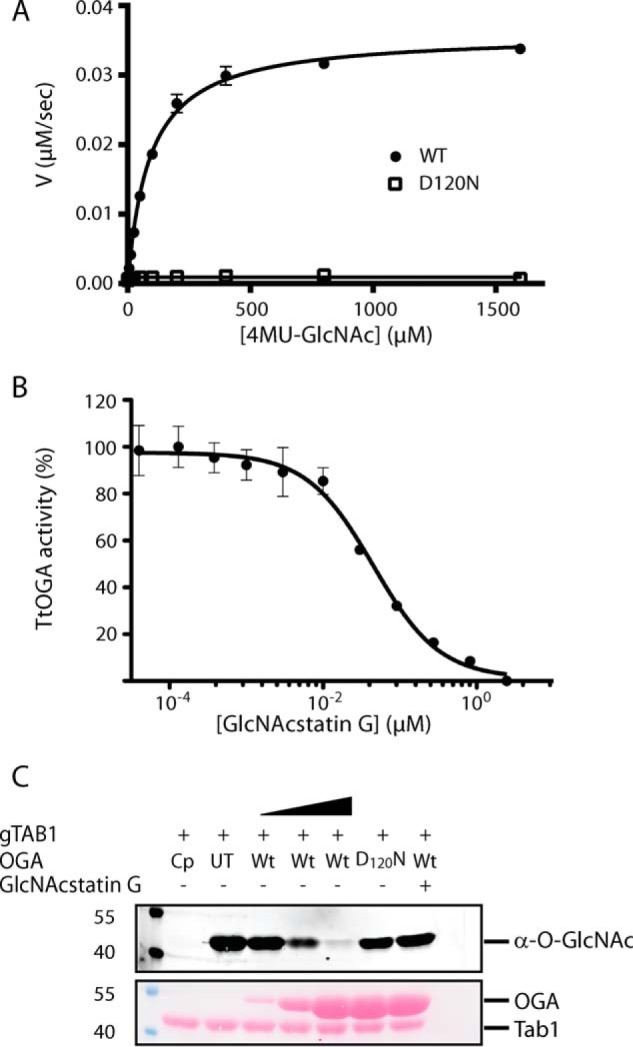
TtOGA is an active O-GlcNAcase. A, steady-state kinetics of the enzymatic activity of the wild type (closed black circles) and D120N (open squares) TtOGA against the pseudosubstrate 4MU-GlcNAc. The initial velocities of the enzymatic reactions (in μm of product/s) in relation to the substrate concentration (in μm) are shown. B, wild type TtOGA subjected to the inhibitor GlcNAcstatin G. Inhibition is shown as percentage of activity of the uninhibited enzyme as a function of inhibitor concentration in μm. The results are representative of three biological repeats, and the error bars represent S.D. C, TtOGA O-GlcNAcase activity on glycosylated hTab1. O-GlcNAcylated hTab1 was treated with an increasing concentration (1, 5, and 15 μm) of the wild type TtOGA (WT), the inactive D120N mutant (15 μm), the wild type enzyme (15 μm) preincubated with GlcNAcstatin G, or wild type CpOGA (1 μm) (Cp) as a positive control. An untreated negative control is shown (UT). O-GlcNAcylated proteins were detected with O-GlcNAc-specific RL-2 antibody. Even loading was tested by staining of the membrane with Ponceau S. Bands corresponding to TtOGA and hTab1 are indicated.
