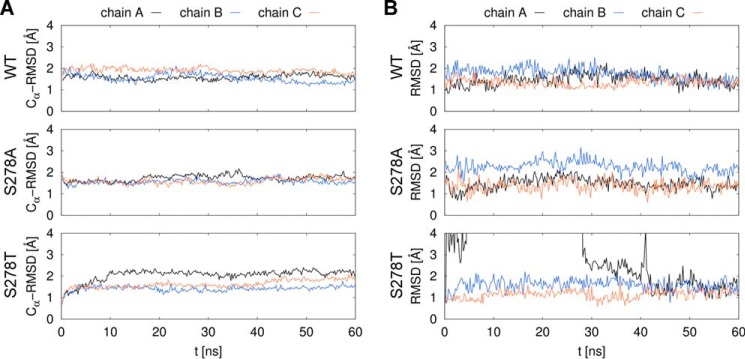
FIGURE 8.
Stability of the protein chains and the substrate molecules in GltPh simulations. Molecular dynamics simulations of 60 ns were performed for the WT and two mutant (S278A, S278T) GltPh proteins. Ser-278 corresponds to Ser-364 in human SLC1A2. Individual transporters in the homotrimer are labeled as chain A, B, and C. Root mean square deviation (RMSD) values of coordinates from the initial state are shown for the protein Cα atoms (A) and the l-aspartate substrate atoms (B). A transient unbinding event of the substrate can be seen in chain A of the S278T system on panel B, after which the substrate returns into a near-native state at around 42 ns.