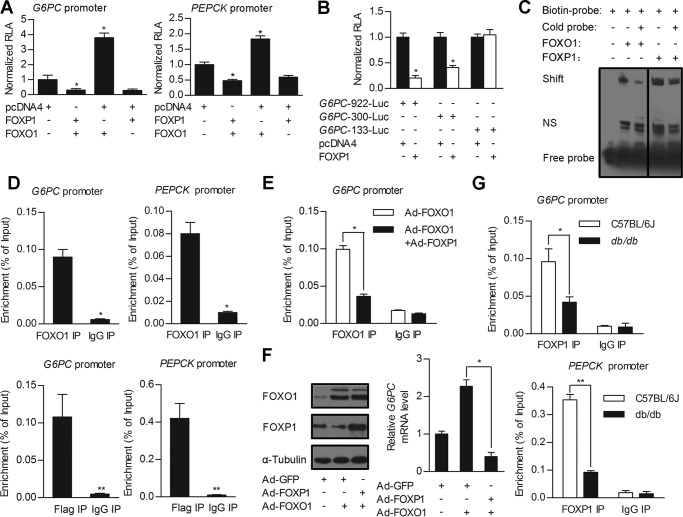
FIGURE 6.
FOXP1 antagonizes activity of FOXO1 on regulation of gluconeogenic genes expression through direct binding to IRE. A and B, luciferase reporter gene assay. A, the G6PC 922-bp and PEPCK promoter-driven luciferase constructs were cotransfected into HepG2 cells, together with pcDNA4 (control) or FOXP1 expression plasmid in the presence/absence of FOXO1 expression plasmid. B, a series of truncated G6PC promoters fused to the luciferase reporter gene were cotransfected into HepG2 cells, together with pcDNA4 (control) or FOXP1 expression plasmids. C, EMSA analysis of HEK293A cells transfected with FOXO1 or FOXP1 expression constructs. The protein extract was incubated with biotin-labeled DNA fragments encompassing IRE of G6PC promoter or cold probe. D, ChIP analysis on primary mouse hepatocytes infected with adenovirus overexpressing FOXO1 and assessed for FOXO1 occupancy on G6PC or PEPCK promoters (top panel). ChIP analysis on primary mouse hepatocytes were infected with adenovirus overexpressing FLAG-tagged FOXP1 and assessed for FOXP1 occupancy on G6PC or PEPCK promoters (bottom panel). E, ChIP analysis on primary mouse hepatocytes infected with Ad-FOXO1 in the presence/absence of Ad-FOXP1, and assessed for FOXO1 occupancy on G6PC promoter. F, Western blotting analysis of FOXP1, FOXO1 protein amounts (left panel), and real-time PCR analysis of the G6PC mRNA level (right panel) in primary mouse hepatocytes infected with Ad-GFP or Ad-FOXP1 in the presence/absence of Ad-FOXO1 following a 2-h treatment with Dex and Fsk. G, ChIP analysis on liver tissues of C57BL/6J and db/db mice, and assessed for endogenous FOXP1 occupancy on G6PC (top panel) or PEPCK promoter (bottom panel). Results are presented as mean ± S.D. of 3 replication experiments. *, p < 0.05; **, p < 0.01.