FIGURE 1.
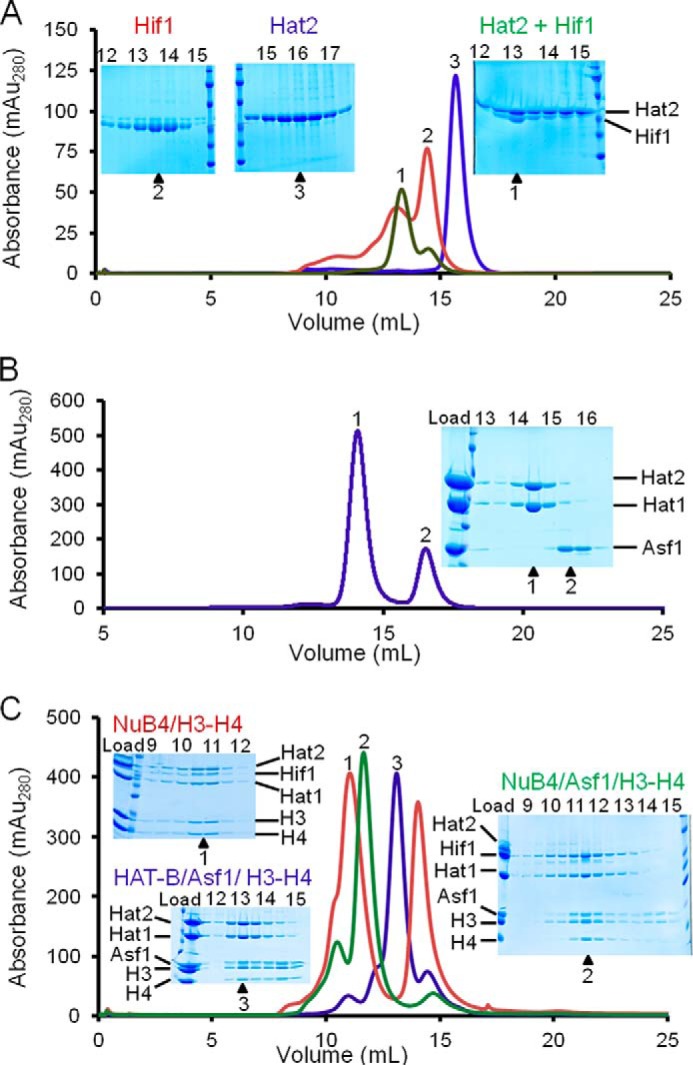
Preparation of Hat1, histone chaperones, and their complexes. Proteins and complexes are analyzed by SEC at protein/complex concentrations of between 1 and 10 μm, and corresponding SDS-PAGE analysis and elution volumes (insets) are indicted. A, Hif1 binding to Hat2 with SEC chromatograms overlaid and color-coded for Hif1 (red), Hat2 (blue), and Hat2 + Hat1 (green) with the corresponding SDS-PAGE analysis. B, SEC of a preformed complex of Asf1, Hat1, and Hat2 with the corresponding SDS-PAGE analysis. C, Hif1 and Asf1 binding modes to HAT-B (Hat1/Hat2) analyzed by SEC and corresponding SDS-PAGE analysis. The following complexes were preformed and analyzed by SEC and SDS-PAGE, and overlaid chromatographs are color-coded as indicated. Trace 1, HAT-B with Hif1 and H3-H4 (NuB4/H3-H4, red); trace 2, HAT-B with Hif1, Asf1, and H3-H4 (NuB4/Asf1/H3-H4, green); trace 3, HAT-B with Asf1 and H3-H4 (HAT-B/Asf1/H3-H4, blue). We note that Hif1 staining on SDS-PAGE appears substoichiometric with other associated Hat1 complexes, suggesting that Hif1 either stains anomalously weakly or partially dissociates from the complex during SEC.
