Abstract
Sleep apnea (SA) leads to metabolic abnormalities and cardiovascular dysfunction. Rodent models of nocturnal intermittent hypoxia (IH) are used to mimic arterial hypoxemias that occur during SA. This mini-review focuses on our work examining central nervous system (CNS) mechanisms whereby nocturnal IH results in increased sympathetic nerve discharge (SND) and hypertension (HTN) that persist throughout the 24-h diurnal period. Within the first 1-2 days of IH, arterial pressure (AP) increases even during non-IH periods of the day. Exposure to IH for 7 days biases nucleus tractus solitarius (NTS) neurons receiving arterial chemoreceptor inputs toward increased discharge, providing a substrate for persistent activation of sympathetic outflow. IH HTN is blunted by manipulations that reduce angiotensin II (ANG II) signaling within the forebrain lamina terminalis suggesting that central ANG II supports persistent IH HTN. Inhibition of the hypothalamic paraventricular nucleus (PVN) reduces ongoing SND and acutely lowers AP in IH-conditioned animals. These findings support a role for the PVN, which integrates information ascending from NTS and descending from the lamina terminalis, in sustaining IH HTN. In summary, our findings indicate that IH rapidly and persistently activates a central circuit that includes the NTS, forebrain lamina terminalis, and the PVN. Our working model holds that NTS neuromodulation increases transmission of arterial chemoreceptor inputs, increasing SND via connections with PVN and rostral ventrolateral medulla. Increased circulating ANG II sensed by the lamina terminalis generates yet another excitatory drive to PVN. Together with adaptations intrinsic to the PVN, these responses to IH support rapid onset neurogenic HTN.
Keywords: intermittent hypoxia, sympathetic nervous system, central nervous system
according to a national sleep foundation study, ∼31% of men and 21% of women in the US are at risk of suffering from sleep apnea (SA) (24). Patients with SA almost invariably present with arterial hypertension (HTN), a major risk factor for acute life-threatening events such as myocardial infarction and stroke (16, 42, 45). A definite cause-and-effect relationship exists between SA-associated HTN and associated cardiovascular events as treatment of SA reduces mean arterial pressure (MAP), sympathetic nerve discharge (SND), and incidents of cardiovascular mortality (4, 5, 34). The etiology of the HTN is not fully understood as it is not feasible to study the onset of the disease in humans; however, available evidence points toward elevated SND as an underlying cause (36, 39). Our group has been investigating early onset neural mechanisms of SA-induced modulation of autonomic control that cause a persistent rise of SND and MAP using an animal model of the hypoxemia that accompanies SA. This model utilizes intermittent exposures to hypoxia during the nocturnal period as first described by the Fletcher laboratory (20, 21).
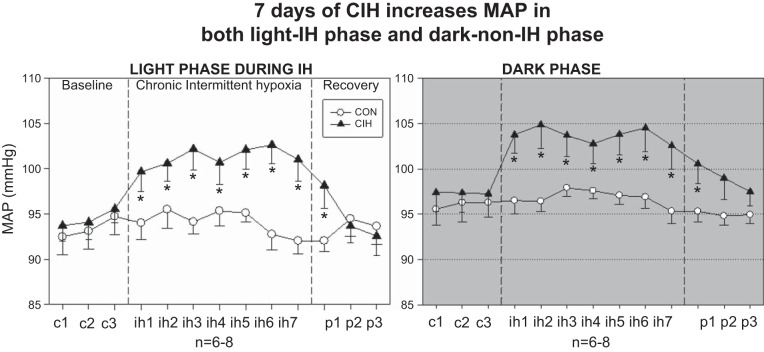
HTN can be induced in rats by chronic exposures to intermittent hypoxia (IH). Of particular relevance is the observation that although patients with SA experience repetitive apnea-induced hypoxemias at night while asleep, their hypertension persists during periods of the day when they are awake and breathing normally. To mimic SA-associated hypoxemias, IH protocols typically expose animals to intermittent hypoxia for only 8 h each day. The significance of this model to human SA is that nocturnal exposures to IH induce a sustained HTN (Fig. 1 presented here and from Ref. 28) that persists during the remaining, 16-h non-IH exposure period of the diurnal period.
Fig. 1.
Effect of 7 days of chronic intermittent hypoxia (CIH) on mean arterial pressure (MAP) and heart rate (HR) during the light phase (left) and the normoxic dark phase (right). All rats were normoxic for 3 days of baseline (c). Data shown for the light phase are mean pressures from 0800 to 1600 during CIH (ih). Following CIH exposure, rats were allowed to recover for 3 days (p). Animals in subsequent experiments were killed on the morning following ih7. CON, control. *P < 0.05 vs. control and CIH; both control and CIH groups n = 6–8 rats. [From Knight et al. (28).]
Our data, as well as those of others, indicate that, as observed in humans with SA (37, 38), exposure to IH results in elevated SND and enhanced arterial chemoreflex sensitivity (20, 23, 25, 47, 49). Furthermore, the IH-induced increase of MAP that occurs in rats is equivalent in magnitude to the fall of MAP observed when SA patients are treated with continuous positive airway pressure to eliminate nocturnal apneas (5, 34). In addition to the deleterious effects of increased MAP, pathological consequences of chronically elevated SND are of critical importance. SA and elevated SND are associated with insulin resistance, hyperinsulinemia, dyslipidemias, activation of the renin-angiotensin system (RAS) activity, increased thrombosis, as well as vascular, cardiac, and renal end-organ dysfunction, damage, and remodeling (16, 43, 65). Similar pathologies occur in rodents following exposures to IH independently of obesity or other comorbid conditions (9, 26, 35, 63). We have utilized the IH model in rats to provide new insights that offer an initial explanation for the paradox posed by Kuniyoshi and Somers (31), “why should a disorder occurring exclusively during sleep elicit sustained increases in blood pressure during wakefulness?”
Our work, as well as that of others, leads us to propose the following model for elevated SND and MAP induced by exposures to IH. In this model, arterial chemoreceptors activate neurons in the nucleus of the solitary tract (NTS) to increase SND and MAP. Elevated renal SND increases circulating ANG II, which acts on ANG II-responsive neurons within the forebrain lamina terminalis. Adaptations within NTS and lamina terminalis primarily act in a feed-forward manner and strengthen sympathoexcitatory drive. Excitatory drives from NTS and lamina terminalis converge within the hypothalamic paraventricular nucleus (PVN), which responds by undergoing an array of adaptations that enhance the signal relayed to sympathetic regulatory neurons in the brainstem and spinal cord.
Of special note is our observation that mechanisms capable of causing persistent sympathoexcitation following IH have a much more rapid onset than presumed. Indeed, we found that persistent increases of SND and MAP induced by IH occur within the first 1–2 days of exposure. This should perhaps not come as a surprise given evidence that increases of SND lasting for several hours can be induced in rats by brief, repeated exposures to hypoxia (17, 18) and in humans by voluntary breath-holding apneas (12, 13, 64). An additional point to emphasize is our finding that exposure of rats to IH is sufficient to model SA-HTN without the necessity of combining IH with hypercapnia (62). This observation was originally reported by Fletcher and colleagues (2, 20, 21) and has since been confirmed in human studies using acute exposures to IH (13, 64).
Elucidating the neural adaptations to IH is important because doing so will likely hold the key to understanding the hypertensive response and, hence, how to improve clinical treatment of SA-associated HTN. We identified several neuronal adaptions to IH that activate and sensitize sympathetic and neuroendocrine function even at times when animals are not experiencing hypoxia. These adaptations directly contribute to HTN in our model. A better understanding of the mechanisms responsible for these adaptations will expand our understanding of how the central nervous system (CNS) responds to IH and may eventually lead to the development of strategies to restore synaptic homeostasis and prevent/reverse neurogenic aspects of SA pathophysiology.
Consistent with other studies using longer duration exposures to IH, we found that after 7 days of IH rats had significantly increased hematocrit and MAP (58). Blockade of ganglionic transmission caused a significantly greater reduction of MAP in rats exposed to IH than control rats, indicating a greater contribution of SND in the support of MAP even at this early stage of IH HTN (58).
Our subsequent studies found that 7 days of IH induced expression of the neuronal plasticity-associated transcription factor FosB in central sites linked to chemoreflex regulation of SND. Prominent among these sites are caudal catecholaminergic (A2) and noncatecholaminergic neurons in the NTS, the rostral ventrolateral medulla (RVLM), and the PVN (28). FosB immunoreactivity was also present in the forebrain lamina terminalis region, which consists of the subfornical organ (SFO), organum vasculum of the lamina terminalis (OVLT), as well as the median preoptic nucleus (MnPO).
The SFO and OVLT are circumventricular organs that project to the MnPO and these structures are critical for mediating responses to circulating ANG II, including thirst, salt appetite, pituitary release of vasopressin, and, importantly for our model, increased SND. The role of these structures in the regulation of SND is key to our interest because of the demonstrated role that ANG II plays in IH-induced HTN (19, 22). This brief review will focus on our findings related to the role of three regions in the central nervous system that are proposed to play major roles in the sympathoexcitatory response to IH: the NTS, the lamina terminalis, and the PVN.
IH and NTS Catecholaminergic Neurons: Activation of CNS Stress Pathways
Our finding of FosB immunoreactivity in the A2 noradrenergic cell group after exposure to IH (28) was intriguing as A2 neurons play an important role in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis (52) and there is evidence for altered HPA axis function in SA patients (6–8, 32). We found that release of ACTH in response to a novel stress (restraint) was enhanced after a 7-day exposure to IH (33). Rats killed after exposure to 35 days of IH had elevated plasma corticosterone (67), further indicating that IH enhances HPA axis function. Recent studies indicate that in addition to its classically defined actions, corticosterone can act within the hindbrain to increase MAP (56) and potentiate the central actions of ANG II to increase MAP (55). To directly assess the contribution of A2 neurons to the IH-induced increase of MAP, we injected an adeno-associated viral (AAV) vector into the caudal NTS that encoded an shRNA against the catecholamine synthetic enzyme tyrosine hydroxylase (TH). We found that TH knockdown in NTS reduced the IH-induced increase of MAP (3) indicating that the elevation of MAP is dependent on production of catecholamines by NTS neurons. Furthermore, knockdown of TH reduced the number of PVN, but not RVLM, neurons expressing FosB after a 7-day exposure to IH, indicating that the A2 input to PVN is activated during IH.
Using an in vitro brain slice preparation to analyze the effects of 7-day IH, we further found that NTS neuronal responses to exogenous application of the excitatory amino acid agonist α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) are enhanced, while responses to N-methyl-d-aspartate (NMDA) are reduced (15). Furthermore, IH exposure also increases the amplitude of glutamatergic miniature excitatory postsynaptic currents resulting from spontaneous release of l-glutamate (Q. Wu and S. Mifflin, unpublished observations). These observations may seem at odds with reports that the amplitude of evoked glutamatergic excitatory postsynaptic currents is reduced in NTS following IH (27). However, it has been suggested that IH reduces the number of active synapses in NTS neurons (1), which could explain the reduced amplitude of tractus-evoked glutamatergic EPSCs in the face of increased postsynaptic sensitivity to l-glutamate.
During IH, NTS neurons not only receive glutamatergic inputs from arterial chemoreceptors but also are exposed to tissue hypoxia. We found that tissue hypoxia evoked an outward KATP current in NTS neurons (66). In many other central neurons, KATP currents provide protection from hypoxic/ischemic injury by reducing neuronal excitability and hence metabolic demand for oxygen. IH exposure reduced KATP current amplitude and expression of KATP channel-associated proteins (66), effectively removing a “brake” on NTS neuronal excitability during hypoxia and promoting exaggerated discharge to a given level of excitatory input.
To summarize, available evidence indicates that IH induces adaptations in NTS neurons that promote increased discharge in response to glutamatergic inputs even in the face of local tissue hypoxia. These adaptations are proposed to be a major mechanism for persistently elevating SND and MAP during IH by enhancing arterial chemoreflexmediated sympathoexcitation.
IH and Lamina Terminalis Neurons: Activation of CNS ANG II Sympathoexcitatory Pathways
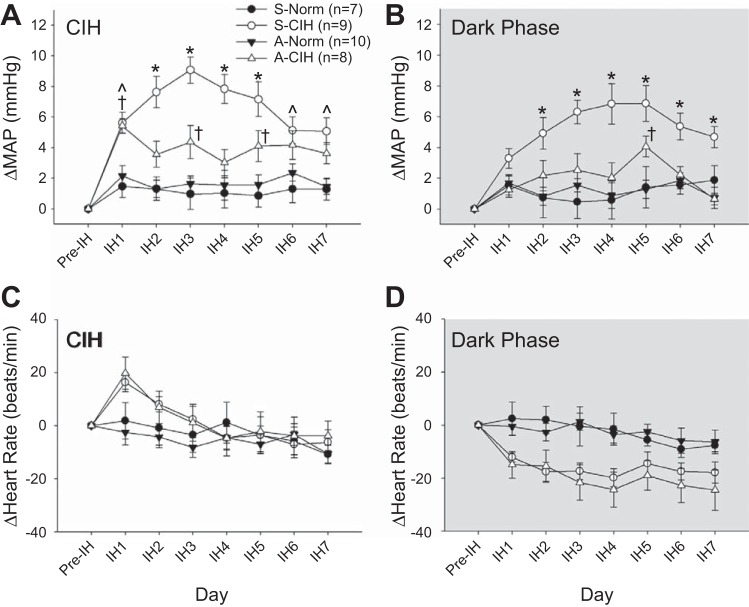
Early work by the Fletcher laboratory demonstrated that IH HTN was dependent on ANG II (19, 22) but did not determine if the ANG II dependence arose from central and/or peripheral sites of action. As previously discussed, exposure to IH significantly increases FosB immunoreactivity in the ANG II-sensing circuitry of the forebrain lamina terminalis, specifically the MnPO, SFO, and OVLT (28). Inhibition of FosB in the MnPO using a virally generated dominant-negative construct significantly attenuated IH HTN, but this effect was selective to the sustained phase HTN that occurs during the normoxic dark cycle (11). Inhibition of FosB in the MnPO also was associated with decreased FosB staining in the PVN and RVLM but not the NTS. There are several candidate genes in the MnPO that are FosB regulated. These include RAS genes encoding ACE1 and AT1a receptors, which could contribute to IH HTN. Evidence supporting a role for CNS effects of ANG II was provided by showing that chronic intracerebroventricular infusions of the AT1 receptor antagonist losartan blocked IH HTN during the normoxic dark cycle (30). Consistent with our previous findings, the antihypertensive effect of losartan was associated with decreased FosB staining in MnPO, PVN, and RVLM. Based on these results, we have adopted an approach for site specific knockdown of the AT1a receptor using shRNA encoding AAV vectors. As expected, knockdown of the ANG II AT1a receptor in the SFO reduced drinking responses to subcutaneously administered ANG II. Moreover, it prevented the development of IH HTN during both the period of nocturnal IH and during the normoxic dark cycle (Fig. 2) (54). Consistent with our earlier observations, FosB staining in the MnPO and PVN were also significantly reduced by AT1a receptor knockdown in the SFO. Our results indicate that activation of the SFO-MnPO pathway during IH is responsible for FosB-mediated changes in gene expression in MnPO that contribute to chronic activation of the PVN. This pathway appears to contribute to the sustained component of IH HTN during normoxia. The results also indicate that chronic activation of the NTS and the increases of MAP during hypoxic episodes are largely independent of the lamina terminalis.
Fig. 2.
Effects of AT1a receptor knockdown in the subfornical organ (SFO) on MAP and HR. The changes from baseline (Pre-IH) are shown in MAP during CIH (A) and dark phase (B; room air breathing) and in HR during CIH (C) and dark phase (D; room air breathing). Data are expressed as means ± SE and analyzed using two-way repeated measures ANOVA followed by Student-Newman-Keuls test. S-Norm = scrambled shRNA + Normoxia; S-CIH = scrambled shRNA + CIH; A-Norm = shRNA for AT1aR + Normoxia; A-CIH = shRNA for AT1aR + CIH. *P < 0.05, S-CIH vs. all other groups; †P < 0.05, A-CIH vs. A-Norm and S-Norm; CIH vs. A-Norm and S-Norm. [From Saxena et al. (54).]
IH and Hypothalamic PVN Neurons: Enhanced Basal Sympathetic Outflow and Reactivity
A brain region that integrates ascending information from the NTS and descending information from the lamina terminalis is the hypothalamic PVN (41). The PVN has been shown to participate in arterial chemoreflex sympathoexcitation (40, 51) and to augment cardiorespiratory outflow via projections to the RVLM after exposure to IH (48). These observations together with evidence that infusion of the ANG II AT1a receptor antagonist losartan into the PVN reduces IH HTN (14) led us to investigate mechanisms whereby preautonomic PVN neurons integrate arterial chemoreceptor and ANG II-sensitive inputs from the NTS and MnPO, respectively. As a critical first step, we established that PVN neuronal activity is essential to maintain IH HTN (58). This was accomplished by inhibiting PVN neurons with the GABAA receptor agonist muscimol, which caused greater reductions of lumbar SND and MAP in IH rats than in normoxic controls. We concluded that neurogenic mechanisms are activated early in the development of IH HTN such that elevated MAP relies on increased sympathetic tonus and ongoing PVN neuronal activity.
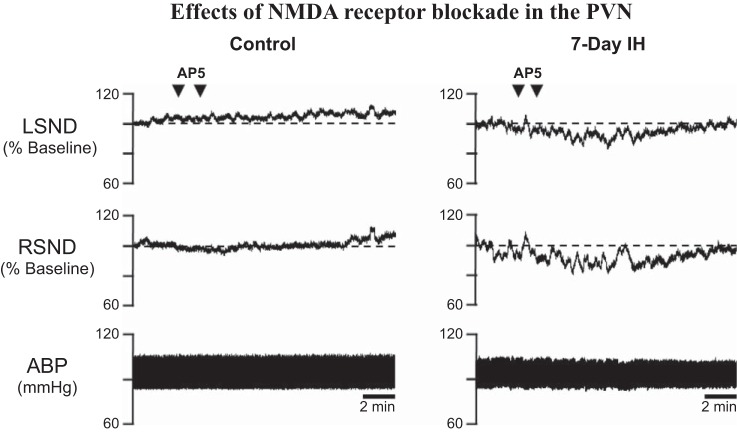
We next determined the role of glutamatergic transmission in PVN-driven support of IH HTN. Whereas AMPA receptor blockade had no effect in normoxic or IH-exposed rats, NMDA receptor blockade reduced SND and MAP only in IH rats (Fig. 3) (G. M. Toney, unpublished observations). Thus 7-day IH increases functional NMDA receptor tone in the PVN to maintain IH HTN. Greater in vivo NMDA receptor tonus could reflect greater synaptic l-glutamate release, reduced l-glutamate clearance increased postsynaptic l-glutamate responsiveness and/or greater PVN neuronal excitability. Functional studies indicate that IH does not change intrinsic excitability of identified presympathetic PVN neurons (Q. H. Chen and G. M. Toney, unpublished observations) but does increase SND responses to PVN microinjections of NMDA and to physiological stimuli (e.g., increases in central hypertonicity) that drive glutamatergic inputs to PVN as discussed in the following section. Together, these findings indicate that synaptic, not intrinsic, adaptations account for early onset PVN activation by IH. Of note is that this conclusion is consistent with evidence that PVN neurons in mice exposed to IH for 35 days show signs of increased glutamatergic NMDA receptor turnover along with reduced NMDA-stimulated production of inhibitory nitric oxide (10).
Fig. 3.
Effects of N-methyl-d-aspartate (NMDA) receptor blockade in the paraventricular nucleus (PVN). Bilateral PVN microinjection of the NMDA receptor antagonist AP5 (3.0 nmol in 50 nl/side) was without effect on lumbar sympathetic nerve discharge (LSND), renal SND (RSND), or arterial blood pressure (ABP) in a normoxic control rat (left) but decreased LSND and RSND in a rat exposed to 7 days of IH (right).
IH Enhances Responses to Homotypic and Heterotypic Stressors
Early work demonstrated that exposure to IH facilitates the MAP response to a brief exposure to hypoxia (20, 23) as observed in SA patients (37, 38, 61). Hence, IH enhances circuit responses to homotypic stress (i.e., hypoxia). We were therefore also interested in determining if IH would enhance responses to heterotypic stressors such as restraint stress and CNS hyperosmolality. We found that the ACTH response to a 30-min period of restraint stress was significantly increased in duration following a 7-day exposure to IH (33). Because IH activates brain regions controlling body fluid homeostasis such as the lamina terminalis and PVN, the effects of internal carotid artery injection of hypertonic saline were tested and determined to increase lumbar SND more in IH-exposed rats than normoxic controls (58). Increased sensitivity to homotypic stress (acute hypoxia) and heterotypic stress (restraint, hypertonicity) in IH-exposed rats suggests that early neuroadaptations among hypoxia sensitive as well as body fluid regulatory neurons could contribute to the initiation of IH HTN.
Sympathoexcitatory Neuroadaptations to IH and Metabolic Syndrome Do Not Interact
Since obesity and metabolic syndrome have been linked to SA and to neurogenic HTN, and because SA adversely affects aerobic exercise capacity (50, 53), we tested the hypothesis that obese rats selectively bred for low aerobic capacity running (LCR) have a greater hypertensive response to IH than lean rats bred for high aerobic capacity running (HCR) (57). First, we compared the contribution of SND to the maintenance of resting MAP using blockade of ganglionic transmission. Despite data indicating that LCR rats were less active and had greater body weight than HCR rats, resting MAP, the contribution of SND to maintenance of MAP, and the hypertensive response to IH were all similar between strains. The contribution of PVN neuronal activity to maintenance of SND and HTN following IH was also compared, and we unexpectedly found that lean HCR rats, not obese LCR rats, showed greater reductions of MAP in response to PVN inhibition. Because LCR-HCR rats are a model metabolic syndrome and not of obesity per se, it is important to stress that greater PVN activation by IH in lean HCR rats might reflect their higher metabolic rate. If so, IH-induced neurogenic HTN in the LCR-HCR model might not be predictive of IH-obesity interactions. Nevertheless, our findings raise the possibility that having low aerobic capacity does not increase vulnerability to sympathoexcitation or neurogenic HTN induced by IH or possibly by SA.
Summary
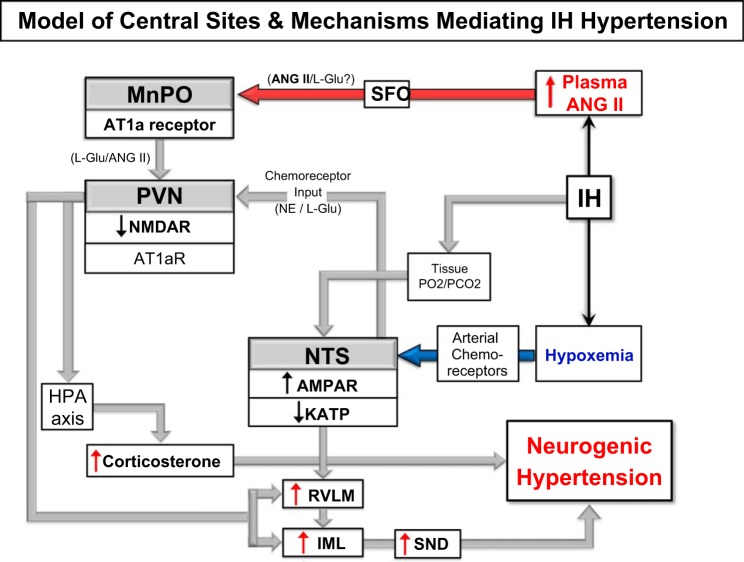
In sum, exposure to nocturnal IH rapidly induces neuroadaptations at multiple sites in the arterial chemoreflex pathway, from the receptor itself (47, 49), to the central sites that integrate the afferent input (NTS), to the central sites mediating and modulating sympathetic outflow (PVN, RVLM) and in the central circuitry involved in sensing and responding to changes in circulating angiotensin II (lamina terminalis). Collectively these functional alterations contribute to persistently elevated SND. The temporal sequencing and time course of adaptations within specific sites is an area worthy of further investigation. Our working model (Fig. 4) holds that NTS neuromodulation increases transmission of arterial chemoreceptor inputs, increasing SND via connections with PVN and RVLM. Increased circulating ANG II sensed by the lamina terminalis generates yet another rapid-onset excitatory drive to PVN and together these adaptations during IH support rapid onset neurogenic HTN. There is evidence that at least some component of the increased SND induced by IH correlates with enhanced expiratory activity (68). The drive to the appropriate respiratory neurons could arise from enhanced discharge of NTS neurons receiving arterial chemoreceptor inputs. While beyond the scope of this review, IH exposure has been documented to alter end-organ and vascular function (44, 46, 59, 60).
Fig. 4.
Model of central pathways involved in the neurohumoral responses to IH. Hypoxic activation of arterial chemoreceptors activates nucleus tractus solitarius (NTS) neurons and via connections to PVN and rostral ventrolateral medulla (RVLM) increases SND. Increased renal SND induces increased circulating ANG II, which is sensed by the lamina terminalis and median preoptic nucleus (MnPO) to generate another excitatory drive to PVN that contributes to IH-induced increases in MAP. Activation of catecholaminergic neurons in caudal NTS stimulates the hypothalamic-pituitary-adrenal (HPA) axis. The model also indicates demonstrated mechanisms within each area that contributes to the ultimate neurogenic hypertension (HTN). During IH, NTS neurons receiving arterial chemoreceptor afferent inputs have been shown to have enhanced responses to exogenous administration of AMPA and reduced KATP channel function. ANG II acts within the SFO, which then excites PVN and RVLM neurons to contribute to the IH-induced neurogenic HTN. The NTS and lamina terminalis are activated by chemoreceptor afferent inputs and ANG II, respectively, during IH and this excitation is relayed to the PVN, which via projections to the RVLM and spinal cord can further augment sympathetic outflow. NTS catecholaminergic inputs to PVN can activate the HPA axis and increased corticosterone can augment the neurogenic HTN. IML, intermediolateral.
Our model of the central pathways involved in the neurohumoral responses to IH proposes that IH HTN is initiated by hypoxic activation of arterial chemoreceptors, activating NTS neurons that project to the PVN and RVLM to increase sympathetic outflow. Increased sympathetic drive to the kidneys leads to increased circulating ANG II, which acts in the lamina terminalis to further augment the discharge of sympathoexcitatory neurons in the PVN. Intermittent hypoxia also sensitizes the HPA axis, which results in increased circulating corticosterone, which may, via a yet undefined mechanisms, contribute to sympathoexcitation. The repetitive, cyclical nature of IH results in persistent and chronic activation of sympathoexcitatory systems. The chronic sympathoexcitation, in concert with alterations at the level of the vasculature, results in persistently elevated blood pressure.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-088052.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W.M., J.T.C., and G.M.T. prepared figures; S.W.M., J.T.C., and G.M.T. drafted manuscript; S.W.M., J.T.C., and G.M.T. edited and revised manuscript; S.W.M., J.T.C., and G.M.T. approved final version of manuscript.
REFERENCES
- 1.Almado CE, Machado BH, Leao RM. Chronic intermittent hypoxia depresses afferent neurotransmission in NTS neurons by a reduction in the number of active synapses. J Neurosci 32: 16737–16746, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao G, Randhawa PM, Fletcher EC. Acute blood pressure elevation during repetitive hypocapnic and eucapnic hypoxia in rats. J Appl Physiol 82: 1071–1078, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Bathina CS, Rajulapati A, Franzke M, Yamamoto K, Cunningham JT, Mifflin S. Knockdown of tyrosine hydroxylase in the nucleus of the solitary tractreduces elevated blood pressure during chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1031–R1039, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 50: 417–423, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 107: 68–73, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bratel T, Wennlund A, Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP). Respir Med 93: 1–7, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Buckley TM, Schatzberg AF. Review: on the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 90: 3106–3114, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Carneiro G, Togeiro SM, Hayashi LF, Ribeiro-Filho FF, Ribeiro AB, Tufik S, Zanella MT. Effect of continuous positive airway pressure therapy on hypothalamic-pituitary-adrenal axis function and 24-h blood pressure profile in obese men with obstructive sleep apnea syndrome. Am J Physiol Endocrinol Metab 295: E380–E384, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol 303: R700–R709, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas G, Chan J, Zhou J, Iadecola C, Pickel VM. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 30: 12103–12112, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham JT, Knight WD, Mifflin SW, Nestler EJ. An essential role for DeltaFosB in the median preoptic nucleus in the sustained hypertensive effects of chronic intermittent hypoxia. Hypertension 60: 179–187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 287: H2054–H2060, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxia. J Appl Physiol 96: 754–761, 2004. [DOI] [PubMed] [Google Scholar]
- 14.da Silva AQ, Fontesa MA, Kanagy NL. Chronic infusion of angiotensin receptor antagonists in the hypothalamic paraventricular nucleus prevents hypertension in a rat model of sleep apnea. Brain Res 1368: 231–238, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Paula PM, Tolstykh G, Mifflin SW. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292: R2259–R2265, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dick TE, Hsieh YH, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol 286: R1121–R1128, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34: 309–314, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher EC, Lesske J, Behm R, Miller CC 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72: 1978–1984, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher EC, Lesske J, Qian W, Miller CC, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol 92: 627–633, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol 86: 298–305, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the National Sleep Foundation Sleep in America 2005 poll. Chest 130: 780–786, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y, Weiss JW. Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol 166: 102–106, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Jun JC, Shin MK, Devera R, Yao Q, Mesarwi O, Bevans-Fonti S, Polotsky VY. Intermittent hypoxia-induced glucose intolerance is abolished by alpha-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab 307: E1073–E1083, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline D, Ramirez-Navarro A, Kunze DL. Adaptive depression in synpatic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: Evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/deltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight WD, Saxena A, Shell B, Nedungadi TP, Mifflin SW, Cunningham JT. Central losartan attenuates increases in arterial pressure and expression of FosB/ΔFosB along the autonomic axis associated with chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1051–R1058, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuniyoshi FH, Somers V. Sleep apnea in hypertension: when, how, and why should we treat? Hypertension 47: 818–819, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Lanfranco F, Gianotti L, Pivetti S, Navone F, Rossetto R, Tassone F, Gai V, Ghigo E, Maccario M. Obese patients with obstructive sleep apnoea syndrome show a peculiar alteration of the corticotroph but not of the thyrotroph and lactotroph function. Clin Endocrinol 60: 41–48, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Ma S, Mifflin SW, Cunningham JT, Morilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coeruleus in response to subsequent immobilization stress. Neuroscience 154: 1639–1647, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNicholas WT. Cardiovascular outcomes of CPAP therapy in obstructive sleep apnea syndrome. Am J Physiol Regul Integr Comp Physiol 293: R1666–R1670, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Moreau JM, Ciriello J. Effects of acute intermittent hypoxia on energy balance and hypothalamic feeding pathways. Neuroscience 253: 350–360, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Narkiewicz K, Somers V. The sympathetic nervous system and obstructive sleep apnea. J Hypertens 15: 1613–1619, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 97: 943–945, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99: 1183–1189, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98: 772–776, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the pressor response to chemoreflex activation in awake rats. Brain Res 895: 167–172, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Front Neuroendocrinol 20: 270–295, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol 293: R1671–R1683, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc 79: 1036–1046, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol 592: 3841–3858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips BG, Somers VK. Hypertension and obstructive sleep apnea. Curr Hypertens Rep 5: 380–385, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 286: H388–H393, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Prabhakar N, Dick TE, Nanduri N, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol 92: 39–44, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Prabhakar NR, Balan KV, Tjoe SS, Martin RJ, LaManna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol 32: 447–449, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Przybylowski T, Bielicki P, Kumor M, Hildebrand K, Maskey-Warzechowska M, Korczynski P, Chazan R. Exercise capacity in patients with obstructive sleep apnea syndrome. J Physiol Pharmacol 58, Suppl 5: 563–574, 2007. [PubMed] [Google Scholar]
- 51.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol 289: R789–R797, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizzi CF, Cintra F, Risso T, Pulz C, Tufik S, de Paola A, Poyares D. Exercise capacity and obstructive sleep apnea in lean subjects. Chest 137: 109–114, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Saxena A, Little JT, Nedungadi TP, Cunningham JT. Angiotensin II type 1 1A receptors in subfornical organ contributes towards chronic intermittent hypoxia associated sustained increase in mean arterial pressure. Am J Physiol Heart Circ Physiol 308: H435–H446, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheuer DA, Bechtold AG. Glucocorticoids potentiate central actions of angiotensin to increase arterial pressure. Am J Physiol Regul Integr Comp Physiol 280: R1719–R1726, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol 286: H458–H467, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Sharpe AL, Andrade MA, Herrera-Rosales M, Britton SL, Koch LG, Toney GM. Rats selectively bred for differences in aerobic capacity have similar hypertensive responses to chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 305: H403–H409, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharpe AL, Calderon AS, Andrade MA, Cunningham JT, Mifflin SW, Toney GM. Chronic intermittent hypoxia increases sympathetic control of blood pressure: role of neuronal activity in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 305: H1772–H1780, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 589: 1463–1476, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104: 110–118, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toney GM, Cunningham JT, Mifflin SW. Early neural adaptations to intermittent hypoxia: Triggers for the pathophysiology of sleep apnea. Recent Advances in Cardiovascular Research: From Sleep to Exercise, edited by Ally A, Maher TJ, Wyss JM. Kerala, India: Transworld Research Network; 2010, chapt. 5, p. 1–22. [Google Scholar]
- 63.Wang Y, Guo SZ, Bonen A, Li RC, Kheirandish-Gozal L, Zhang SX, Brittian KR, Gozal D. Monocarboxylate transporter 2 and stroke severity in a rodent model of sleep apnea. J Neurosci 31: 10241–10248, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol 91: 1555–1562, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034–2041, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1555–R1562, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zoccal DB, Bonagamba LG, Antunes-Rodrigues J, Machado BH. Plasma corticosterone levels is elevated in rats submitted to chronic intermittent hypoxia. Auton Neurosci 134: 115–117, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Zoccal DB, Simms AE, Bonagamba LGH, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586: 3253–3265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]