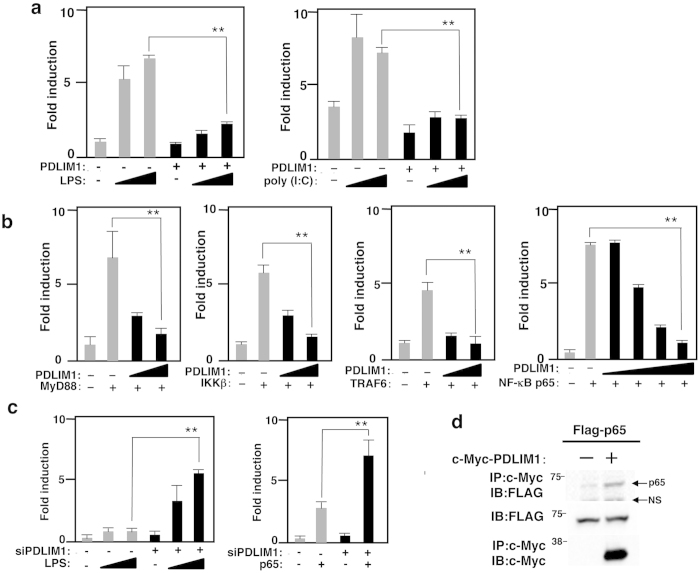
Figure 2. PDLIM1 binds to p65 and suppresses NF-κB signaling.
(a) Luciferase activity in MEF transfected with a pGL4-NF-κB luciferase reporter construct along with or without a plasmid encoding PDLIM1, then left untreated or treated with LPS (2 and 10 ng/ml) or poly(I:C) (10 and 50 μg/ml) for 5 hr. Data are representative of at least three independent experiments. (b) Luciferase activity in 293T cells transfected with an ELΑΜ−1 luciferase reporter construct (ELAM-1-luc) with or without plasmids encoding MyD88, TRAF6, IKKβ or p65 in absence or presence of increasing amounts (wedge) of PDLIM1. (c) Luciferase activity in MEF first transfected with control or PDLIM1-specific siRNA and then with a ELΑΜ−1 luciferase reporter construct, which were then stimulated without or with LPS for 6 hours (left), or MEF first transfected with control or PDLIM1-specific siRNA and then with a ELΑΜ−1 luciferase reporter construct without or with the plasmid encoding p65 (right). Data are representative of at least three independent experiments and are shown as means ± SD. **P < 0.01. (d) PDLIM1 interacts with the p65 subunit of NF-κB. 293T cells were transfected with a FLAG-p65 expression plasmid along with or without PDLIM1. The lower bands in top blot are non-specific bands (NS). Whole cell extracts were immunoprecipitated with anti-c-Myc, and immunoblotted with anti-FLAG. Western blots are representative of at least three independent experiments.