Abstract
The goal of recurrent ovarian cancer (ROC) treatment is no longer just palliation, but prolonging survival. This is usually through administering a new line of chemotherapy at each relapse. A novel treatment sequencing strategy to achieve this is through the intercalation of an effective non-platinum alternative, in between platinum-based therapies. Trabectedin in combination with pegylated liposomal doxorubicin (PLD) has been fully available privately in the UK since 2009 for treating patients with ROC. A single institution's experience with the trabectedin + PLD combination, as a non-platinum/non-taxane alternative, to intercalate between platinum-based therapies is reported here. To date 6 patients have been successfully treated with trabectedin + PLD at Broomfield Hospital, Chelmsford, Essex. Here we describe a new, practice-changing treatment approach in a real-life case study of a heavily-treated patient with advanced ROC treated with trabectedin + PLD at fourth-line and then subsequently rechallenged at seventh-line; with treatment continuing until disease progression.
Keywords: Recurrent ovarian cancer, Trabectedin
Real-life patient experience: UK case presentation
A 49-year old woman presented in October 2007 with abdominal bloating, pain, and obvious ascites. After imaging data was obtained, in December 2007 the patient underwent total abdominal hysterectomy (TAH)/bilateral salpingo-oophorectomy (BSO) and debulking surgery to remove as much of the tumour as possible. After surgery the patient was diagnosed with serous ovarian adenocarcinoma, stage III C.
First-line chemotherapy was the recommended taxol/carboplatin combination standard-of-care for 3 cycles, which finished in March 2008. In June 2010 the patient relapsed and there was recurrence of disease, presenting as rapidly accumulating ascites.
The patient was rechallenged with platinum second-line; treatment was taxol/carboplatin for 6 cycles, which was completed in October 2010. The patient responded well to treatment with the ascites disappearing. The patient then agreed to participate in a phase II study investigating the CA-125 doubling time in patients with advanced disease treated with tamoxifen citrate [1].
In April 2011 there was recurrence of disease, and in August 2011 the patient received trabectedin + PLD combination for 5 cycles as fourth-line therapy. The patient achieved a good clinical response according to CA-125 measurement, and the chemotherapy was stopped given the good clinical status of the patient.
In January 2012 the patient experienced disease progression and received subsequent treatment with gemcitabine/carboplatin/bevacizumab combination therapy for 6 cycles, plus bevacizumab 15 mg/kg every 3 weeks as maintenance therapy.
In June 2013 the patient experienced further disease progression and received taxol/carboplatin as weekly treatment for 6 cycles until January 2014, after which the patient's disease was stable.
Disease recurrence occurred in March 2014, with ascites, para-aortic lymph node involvement, and peritoneal disease. The patient was subsequently re-challenged with trabectedin + PLD as seventh-line of chemotherapy. The patient responded clinically according to CA-125 measurement (Fig. 1a), and CT imaging showed a clinical response and improvement in symptoms (Fig. 1b,c).
Fig. 1.
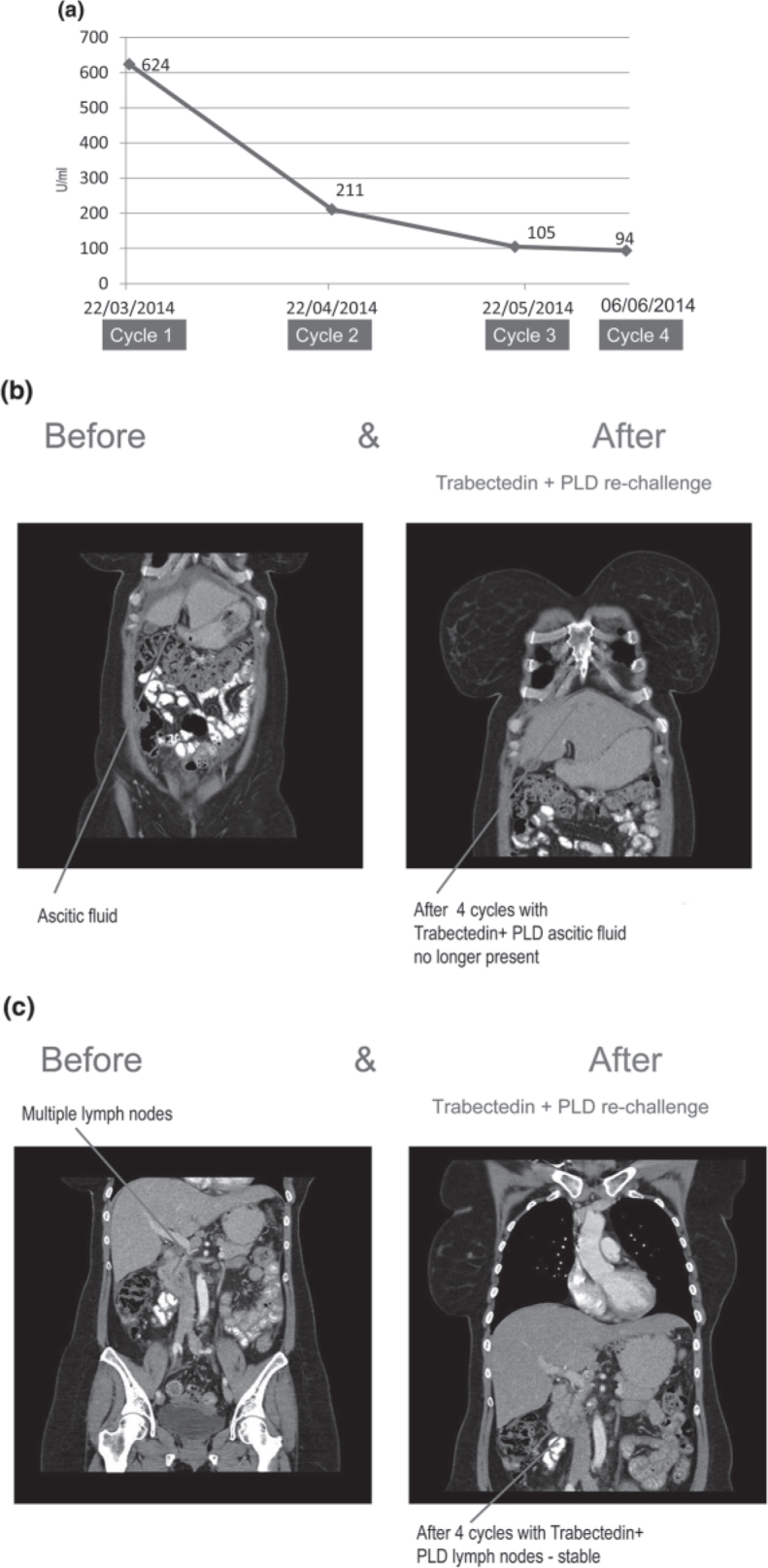
(a) CA-125 measurement following treatment with trabectedin + PLD. (b) CT images of the patient after 4 cycles of trabectedin + PLD showing improvement in symptoms (ascitic fluid). (c) CT images of the patient after 4 cycles of trabectedin + PLD showing changes in lymph nodes.
The dose of trabectedin + PLD was adjusted for this patient due to grade 3 fatigue. The trabectedin dose was reduced from 1.1 to 0.9 mg/m2, and PLD was reduced from 30 to 25 mg/m2. The management plan for this patient is to continue treatment with trabectedin + PLD until disease progression. The patient has finished 11 cycles so far and is still in remission, and continues to do well.
In summary, this case study describes a new concept in the management of a ROC patient who has received 7 different lines of chemotherapy and has experienced recurrent disease. Trabectedin + PLD combination was effective as a non-platinum/non-taxane alternative to intercalate between platinum-based therapies. In fact, trabectedin has been administered for 6 or more cycles in 52% of patients treated with the combination dose and schedule respectively. The combination regimen has been used for up to 21 cycles, and no cumulative toxicities have been observed in patients treated with multiple cycles [2]. So whilst doxorubicin is limited by its cumulative dose due to cardiotoxicity, trabectedin plus PLD can be used to disease progression. In the case study described here the patient is doing well, and will continue maintenance treatment with trabectedin + PLD, with repeat ECHO every 12 weeks, until disease progression.
Practical considerations with trabectedin plus PLD treatment
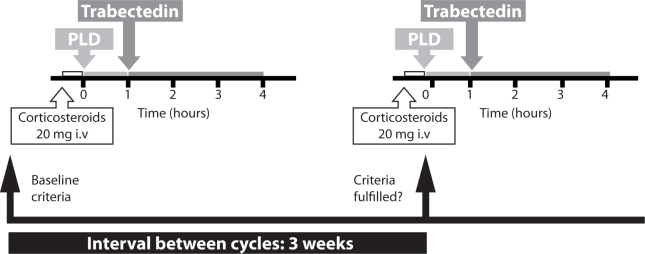
As with all cytotoxic regimens there are some practical considerations to treating patients with trabectedin + PLD. Trabectedin is administered every three weeks as a 3-hour intravenous (i.v.) infusion at a dose of 1.1 mg/m2, immediately after PLD 30 mg/m2, for the treatment of ovarian cancer (Fig. 2) [3]. The following steps must be taken when administering the combination [2]:
-
•
All patients must receive corticosteroids e.g. 20 mg of dexamethasone i.v. 30 minutes prior to PLD
-
•
60 minute i.v. infusion of PLD 30 mg/m2. Intravenous administration through a central venous line is strongly recommended
-
•
3-hour i.v. infusion of trabectedin 1.1 mg/m2, immediately after PLD, every 3 weeks through a central venous line
Fig. 2.
Schematic showing how to administer trabectedin + PLD.
As with other cytotoxic regimens, monitoring of patients receiving trabectedin + PLD is recommended (Box 1).
Box 1. Recommended blood tests for patients receiving trabectedin + PLD.
-
•
Neutrophils
-
•
Platelets
-
•
Bilirubin
-
•
Alkaline phosphatase
-
•
Transaminases ALT and AST
-
•
CPK
-
•
Haemoglobin
Test to be performed weekly during the first two cycles, and at least once between treatments in subsequent cycles.
The patient must satisfy the following criteria for initiation of treatment and prior to each re-treatment:
-
•
Absolute neutrophil count (ANC) ≥1,500/mm3
-
•
Platelet count ≥100,000/mm3
-
•
Bilirubin ≤ upper limit of normal (ULN)
-
•
Alkaline phosphatase ≤2.5 × ULN (consider hepatic isoenzymes 5-nucleotidase or gamma glutamyl transpeptidase (GGT) to determine whether the elevation is of hepatic or osseous origin.
-
•
Albumin ≥25 g/l.
-
•
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤2.5 × ULN
-
•
Serum creatinine ≤1.5 mg/dL (≤132.6 μmol/L) or creatinine clearance ≥60 mL/min
-
•
Creatine phosphokinase (CPK) ≤2.5 × ULN
-
•
Haemoglobin ≥9 g/dl
Otherwise treatment should be delayed and/or reduced for up to 3 weeks until the criteria are met, as highlighted in the case study above (Box 2).
Box 2. Dose modification schedule for trabectedin + PLD [2].
| Trabectedin | PLD* | |
|---|---|---|
| Starting dose | 1.1 mg/m2 | 30 mg/m2 |
| First reduction | 0.9 mg/m2 | 25 mg/m2 |
| Second reduction | 0.75 mg/m2 | 20 mg/m2 |
See the PLD SmPC for more detailed information on PLD dose adjustments [4].
Conclusions
The goal of ROC treatment is no longer just palliation, but prolonging survival. This is achieved by administering a new line of chemotherapy at each relapse. An additional approach may be the administration of maintenance regimens. A single institution's experience with trabectedin + PLD combination, as a non-platinum/non-taxane alternative to intercalate between platinum-based therapies is reported here. A real-life case study is presented of a heavily-treated patient with advanced disease who was treated with trabectedin + PLD fourth-line and subsequently rechallenged at seventh-line, who continues to do well on treatment. This case study demonstrates the utility of the combination at any line of relapse in ROC patients with advanced disease.
Conflict of Interest Statement
Professor Tahir received speaker fees from PharmaMar for his participation at the BGCS 2014 symposium. Professor Tahir is responsible for the content of this article. Patricia Howard Associates received fees from PharmaMar to provide editorial assistance.
References
- 1.http://clinicaltrials.gov/show/NCT00305838
- 2.Yondelis SmPC Accessed October 2014 [https://www.medicines.org.uk/EMC/ingredient/2054/trabectedin]
- 3.Monk BJ, Herzog TJ, Kaye SB. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28:3107–3114. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]
- 4.Pegylated liposomal doxorubicin (Caelyx) SmPC. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000089/WC500020180.pdf]