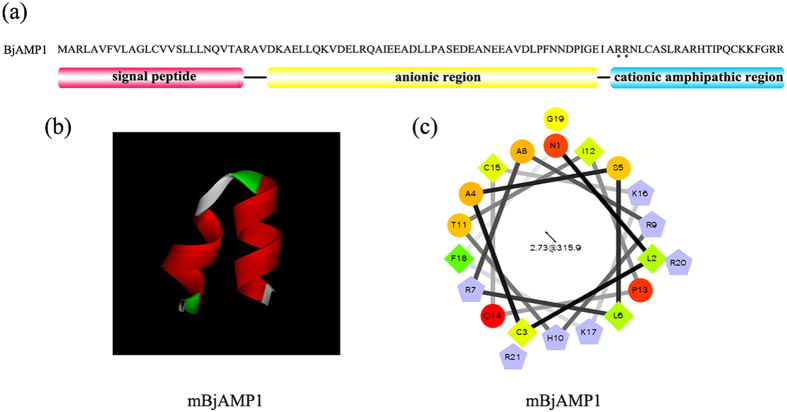
Figure 1. Amino acid composition distribution, 3D molecular modeling and helical wheel projection.
(a) Amino acid composition distribution of BjAMP1. N-terminus is a signal peptide of 24 amino acid residues, followed by an anionic-rich region and a cationic-rich C-terminus extension. The putative dibasic cleavage site (75RR76) is indicated by (*). (b) 3D structure of mBjAMP1. (c) The helical wheel projection of mBjAMP1, demonstrating amphipathic potential of the peptide structure. Residues are numbered starting from the N-terminus. Hydrophilic residues are presented as circles, hydrophobic residues as diamonds, potentially negatively charged as triangles, and potentially positively charged as pentagons. Hydrophobicity is color coded: the most hydrophobic residue is green, and the amount of green is decreasing proportionally to the hydrophobicity, with zero hydrophobicity coded as yellow. Hydrophilic residues are coded red with pure red being the most hydrophilic (uncharged) residue, and the amount of red decreasing proportionally to the hydrophilicity. The potentially charged residues are light blue.