Structured Abstract
Objectives
To determine if Amino-terminal Pro B-type Natriuretic Peptide (NT-proBNP) has different diagnostic and prognostic utility in patients with renal dysfunction.
Background
Patients with renal dysfunction have higher NT-proBNP, which may complicate interpretation for diagnosis of acute decompensated heart failure (ADHF) or prognosis.
Methods
We searched MEDLINE and EMBASE through August 2014 for studies with a subgroup analysis by renal function of the diagnostic or prognostic ability of NT-proBNP.
Results
For diagnosis, nine studies were included with 4,287 patients and 1,325 ADHF events. Patients were mostly divided into sub-groups with and without renal dysfunction by an estimated glomerular filtration rate of 60 ml/min/1.73m2. In patients with renal dysfunction, the area under the curve (AUC) for NT-proBNP ranged from 0.66 to 0.89 with a median cut-point of 1980 pg/ml, while the AUC ranged from 0.72 to 0.95 with a cut-point of 450 pg/ml in patients with preserved renal function. For prognosis, 30 studies with 32,203 patients were included, and mortality in patients with renal dysfunction (25.4%) was twice that of patients with preserved renal function (12.2%). The unadjusted pooled risk ratio (RR) for NT-proBNP and mortality was 3.01 (95% CI, 2.53-3.58) in patients with preserved renal function and was similar in patients with renal dysfunction (3.25 [CI, 2.45-4.30]). Upon meta-regression, heterogeneity was partially explained if patients with heart failure or coronary artery disease were enrolled.
Conclusions
NT-proBNP retains utility for diagnosis of ADHF in patients with renal dysfunction with higher cut-points. Elevated NT-proBNP confers a worse prognosis regardless of renal function.
Keywords: Amino-terminal Pro B-type Natriuretic Peptide, Renal Dysfunction, Diagnosis, Prognosis
Introduction
The measurement of N-terminal pro B-type natriuretic peptide (NT-proBNP) is an accepted diagnostic strategy to clarify whether a patient presenting with dyspnea has acute decompensated heart failure (ADHF) and is a useful prognostic marker for mortality in a broad spectrum of patients 1-3. Incorporation of NT-proBNP in clinical management decreases the cost of medical care and improves clinical outcomes 4-6.
B-type natriuretic peptides are a family of proteins secreted by the ventricles during ventricular wall stretch and are cleaved into a biologically active fragment, B-type natriuretic peptide (BNP), and a biologically inactive fragment, NT-proBNP. It is known that BNP increases natriuresis, diuresis and vasodilation, although there is uncertainty regarding processing and clearance of natriuretic peptides 7. Important steps such as post-translational glycosylation and cleavage by the proteases, furin and corin, are likely deranged in heart failure8-10. While it is commonly believed that NT-proBNP is more reliant on renal excretion than BNP, there is evidence that the kidneys clear both hormones equally 11. Furthermore, mass spectroscopy data has shown that commercial BNP and NT-proBNP assays measure the target peptides as well as precursor molecules and breakdown products12.
Important issues remain about how to use clinical NT-proBNP assays appropriately in the clinical setting. Since over 50% of patients with a diagnosis of ADHF suffer from decreased glomerular filtration rate (GFR), physicians encounter this clinical conundrum frequently 13,14. It would be clinically valuable to clarify if commercially available NT-proBNP assays are diagnostically and prognostically useful in patients with renal insufficiency.
We sought to establish the performance of NT-proBNP for diagnosis of ADHF and prognosis in patients with renal dysfunction by undertaking a systematic review and meta-analysis of the literature. Our study consists of two separate questions. First, does renal dysfunction alter the diagnostic ability of NT-proBNP to detect ADHF? Second, does renal dysfunction alter the prognostic ability of NT-proBNP?
Methods
This study was performed in accordance with published guidelines for systematic review and meta-analysis of observational studies 15.
Study Selection
A study was eligible for inclusion if it included at least 50 patients greater than 18 years of age. For our first question, concerning if renal dysfunction impacts the diagnostic ability of NT-proBNP for ADHF, a study was eligible if the outcome was diagnosis of ADHF, and included a sub-group analysis by kidney function (either by serum creatinine, serum cystatin C, estimated GFR or measured GFR). For our second question, concerning if renal dysfunction impacts the prognostic ability of NT-proBNP, a study was eligible if the outcome was either all-cause mortality, cardiac mortality or major adverse cardiac events (MACE) and included a sub-group analysis by kidney function. For both questions, we searched for acute and chronic renal insufficiency.
Data Sources and Searches
We preformed two separate searches in Ovid Medline and Ovid Embase from inception (1946 and 1974, respectively) until August 2014 based upon our two questions. An example of the full search criteria is included in the supplementary materials (Online Table 1). We additionally searched the Science Citation Index Expanded on Web of Science and reviewed the references of all selected studies. There was no language restriction.
Outcome Measures
For our first and second question, area under the curve (AUC), sensitivity, specificity, positive or negative predictive value were acceptable outcome measures. We additionally included studies that reported hazard ratio (HR), odds ratio (OR), or relative risk (RR) for our second question.
Data Extraction and Quality Assessment
Two reviewers decided if studies were eligible for inclusion (J.A.S. and S.G.C.). Two reviewers (J.A.S. and D.G.M.) independently extracted information using a standardized data collection sheet and final data were decided by consensus. As multiple studies did not provide complete data, we contacted the authors to complete a data sheet of summary information. For studies concerning diagnosis, quality was assessed by the QUADRAS criteria 16; while for prognosis, quality was assessed by the Hayden criteria 17.
Data Synthesis and Analysis
For the analysis regarding effect modification of renal function on diagnostic ability, we used the method developed by Moses and colleagues 18 to produce separate summary receiver operator curves (ROC) for patients with preserved and diminished renal function. This method does not allow estimation of accurate confidence intervals or summary AUC; thus, we examined if there was effect modification by comparing the summary ROCs visually.
For assessing effect modification of renal function on prognostic value, we conducted separate analyses for patients with preserved and diminished renal function, and evaluated the difference between the pooled risk ratios with a two-sample Z test. Crude and adjusted effect estimates were pooled separately to calculate risk ratios using techniques that accounted for within- and between- study heterogeneity (random effects method of DerSimonian and Laird) and were weighted by their inverse variance. If a study provided both a crude and adjusted effect estimate, it was included in both types of analysis. We formally assessed heterogeneity of effects between studies with the Cochran I and τ2 statistics.
If significant heterogeneity was present as indicated by the τ2 statistic, we undertook meta-regression to evaluate for sources of heterogeneity. We used a mixed-effects linear regression model where the random effects were estimated the DerSimonian and Laird estimator and studies were weighted by the inverse variance method. Moderators were pre-specified and were if the study population included patients with CAD versus other co-morbidities, patients with HF versus other co-morbidities, inpatients versus outpatients, renal function estimate (estimated GFR versus other/serum cystatin C versus other), NT-proBNP cut-points (same cut-points for patients with preserved and diminished renal function versus different cut-points) and NT-proBNP assay (Roche versus other). Since there were few studies, each moderator was analyzed individually and we applied a Bonferroni correction factor to create a study wide alpha level of 0.05.
Meta-analysis of diagnostic data and meta-regression were conducted in R v 3.1.2 19,20. The remainder of the analysis was conducted in RevMan v 5.3.
Results
We identified 4012 studies eligible for review after excluding 657 duplicate citations. After title and abstract screening, we identified 236 articles for full text review. Nine articles were eligible to evaluate diagnostic performance and 30 articles were eligible to evaluate prognostic performance (Online Figure 1). Six studies for diagnosis and sixteen studies for prognosis required further information from the authors, and we received responses five authors. After contacting the authors, five studies for diagnostic performance and 17 studies on prognostic ability had complete quantitative data sufficient for meta-analysis.
Study Characteristics Regarding Diagnosis
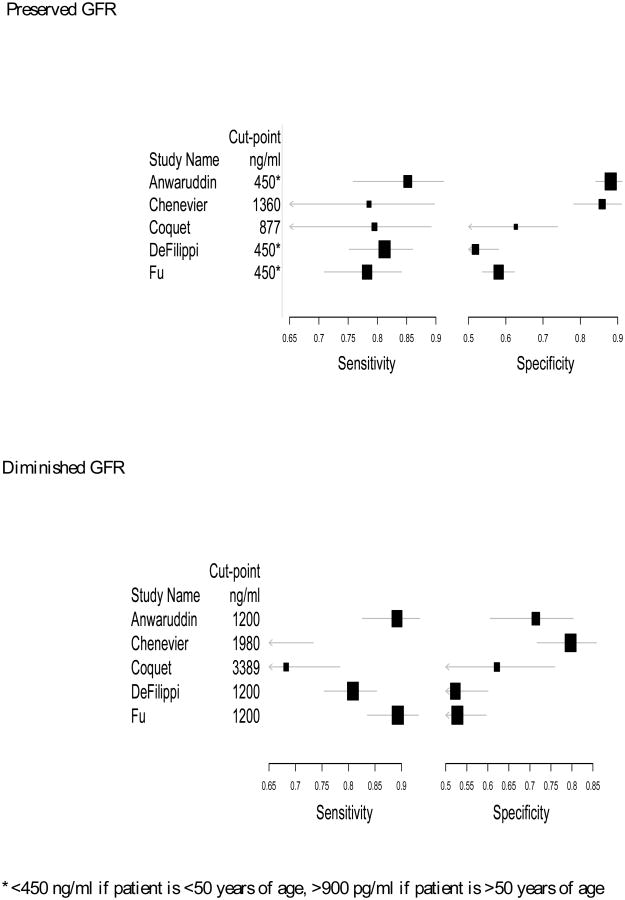
The study and patient characteristics for diagnosis of ADHF are depicted in Table 1A and the summary data are presented in Table 1B. The majority of the studies were conducted in the emergency department setting 21-25. Two studies used the standard “Januzzi cut-offs”, which indicates a NT-proBNP level > 450 pg/ml in patients less than 50 years of age and > 900 pg/ml in patients greater than 50 years of age 21,24, while the remaining studies reported the optimal cut-point from an ROC analysis from their cohort.
Table 1A. Study Characteristics for Diagnosis of Acute Decompensated Heart Failure using NT-proBNP.
| Author, Year (Ref)* | Setting | Sample | # | # ADHF (%) | Men | Age (years) | Quality† | Renal Exclusion | Assay | Renal Function Estimate |
|---|---|---|---|---|---|---|---|---|---|---|
| Anwaruddin, 2006 1 | ER | SOB | 599 | 209 (35) | 57 | 62 | Good | sCr>2.5 | Roche | MDRD |
|
| ||||||||||
| Chenevier-Gobeaux, 2005 2 | ER | SOB | 381 | 115 (30) | NR | 79 | Good | NR | Roche | MDRD |
|
| ||||||||||
| Colak, 2014 3 | ER | SOB | 132 | NR | 52 | 73 | Mod | None | Siemens | MDRD |
|
| ||||||||||
| Coquet, 2008 4 | Inpt | ICU | 198 | 102 (52) | 66 | 67 | Good | ESRD | Roche | MDRD |
|
| ||||||||||
| DeFilippi, 2007 5 | ER | SOB | 831 | 437 (53) | 46 | 66 | Good | None | Roche | MDRD |
|
| ||||||||||
| Fu, 2013 6 | Inpt | CAD | 999 | 306 (31) | 91 | 86 | Mod | None | Siemens | MDRD |
|
| ||||||||||
| Gorrison, 2007 7 | ER | SOB | 80 | 40 (50) | 54 | 74 | Good | None | Roche | MDRD |
|
| ||||||||||
| Lefebvre, 2008 8 | Inpt | ICU | 100 | 16 (16) | 59 | 53 | Mod | NR | Dade Behring | sCr |
|
| ||||||||||
| Park, 2010 9 | ER | SOB | 967 | 100 (10) | 54 | 62 | Mod | NR | Roche | CG |
ER, emergency room; Inpt, inpatient; CAD, coronary artery disease; SOB, shortness of breath; ICU, intensive care unit; MDRD, Modified Diet in Renal Disease; sCr, serum creatinine (mg/dl); CG, Cockcroft-Gault; Mod, moderate; ESRD, end-stage renal disease; NR, not reported
References located in Online Supplementary material.
Quality assessed by QUADRAS criteria16
Table 1B. Summary Data for Diagnosis of Acute Decompensated Heart Failure using NT-proBNP.
| Author, Year | Renal Function Category | # | # ADHF (%) | AUC | Cut-point (pg/ml) | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Anwaruddin, 2006 | eGFR>=60 | 393 | 81 (21) | 0.95 | 450* | 0.85 | 0.88 |
|
| |||||||
| <60 | 206 | 129 (63) | NR | 1200 | 0.89 | 0.72 | |
|
| |||||||
| Chenevier-Gobeaux, 2005 | eGFR 60-89 | 141 | 28 (20) | 0.85 | 1360 | 0.77 | 0.86 |
|
| |||||||
| 30-59 | 187 | 64 (34) | 0.73 | 1980 | 0.62 | 0.80 | |
| 15-29 | 41 | 20 (49) | 0.80 | 6550 | 0.82 | 0.79 | |
|
| |||||||
| Colak, 2014 | eGFR 30-90 | NR | NR | 0.72 | 3839 | 0.68 | 0.75 |
| <90 | 94 | NR | 0.66 | 3670 | 0.68 | 0.60 | |
|
| |||||||
| Coquet, 2008 | eGFR >=60 | 98 | 39 (40) | 0.76 (CI 0.69-0.83) | 877 | 0.80 | 0.63 |
|
| |||||||
| <60 | 100 | 63 (63) | 0.74 (CI 0.64-0.84) | 3389 | 0.69 | 0.62 | |
|
| |||||||
| DeFilippi, 2007 | eGFR >=60 | 438 | 197 (45) | 0.74 (CI 0.70-0.79) | 450* | 0.81 | 0.52 |
|
| |||||||
| <60 | 393 | 240 (61) | 0.66 (CI 0.60-0.71) | 1200 | 0.81 | 0.49 | |
|
| |||||||
| Fu, 2013 | eGFR>=60 | 641 | 147 (23) | 0.72 (CI 0.67-0.77) | 298 | 0.78 | 0.58 |
|
| |||||||
| <60 | 358 | 159 (44) | 0.75 (CI 0.69-0.80) | 436 | 0.89 | 0.53 | |
|
| |||||||
| Gorrison, 2007 | eGFR>=60 | 39 | NR | 0.78 | 1118 | 0.85 | 0.73 |
|
| |||||||
| <60 | 41 | NR | 0.70 | 2592 | 0.70 | 0.64 | |
|
| |||||||
| Lefebvre, 2008 | sCr<1.6 | 75 | NR | 0.69 (SE 0.08) | NR | NR | NR |
|
| |||||||
| 1.6-2.8 | 14 | NR | 0.66 (SE 0.10) | NR | NR | NR | |
| >2.8 | 15 | NR | 0.61 (SE 0.15) | NR | NR | NR | |
|
| |||||||
| Park, 2010 | Systolic HF eGFR>=60 | NR | 45 | 0.92 | 418 | 0.84 | 0.84 |
|
| |||||||
| eGFR<60 | NR | 55 | 0.83 | 1981 | 0.78 | 0.78 | |
|
| |||||||
| Diastolic HF eGFR>=60 | NR | 36 | 0.89 | 276 | 0.83 | 0.83 | |
|
| |||||||
| eGFR<60 | NR | 37 | 0.84 | 1733 | 0.78 | 0.76 | |
eGFR, estimated GFR (1.73/ml/m2); sCr, serum creatinine (mg/dl); AUC, area under the curve; CI, confidence interval; SE, standard error; NR, not reported.
450 indicates >450 pg/ml in patients less than 50 years of age and >900 pg/ml in patients greater than 50 years of age (i.e. Standard Januzzi cut-points).
Study Characteristics Regarding Prognosis
The study and patient characteristics for prognosis are depicted in Table 2A and the summary data for studies that provided quantitative data by sub-group are presented in Table 2B for studies pertaining to prognosis. Follow-up for events was evaluated over a range of time intervals in the studies, spanning from 2 months to 10 years. The studies enrolled patients with various medical co-morbidities, although the most common were HF and CAD. In over half of the studies, the absolute event rates were two-fold higher in patients with renal dysfunction than patients without renal dysfunction.
Table 2A. Study Characteristics for Prognosis.
| Author, Year (Ref)* | Setting | Sample | Design | # Center | # | # Events (%) | Men (%) | Age (yrs) | Quality† | Renal Exclusion | Assay | Renal Function Estimate | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alehagen, 2009 10 | Outpt | HF | Cohort | 1 | 464 | 138 (30) | 52 | 73 | Poor | None | Roche | Cystatin C | Mortality |
|
| |||||||||||||
| Anwaruddin, 2006 1 | ER | SOB | Cohort | 1 | 599 | 30 (5) | 57 | 62 | Good | sCr>2.5 | Roche | MDRD | Mortality |
|
| |||||||||||||
| Apple, 2007 11 | ER | ACS | Cohort | 1 | 457 | NR | 57 | 57 | Mod | NR | Roche | MDRD | Mortality |
|
| |||||||||||||
| Astor, 2008 12 | Outpt | CKD | RCT | 21 | 994 | 134 (14) | 61 | 55 | Good | GFR>65, <20 | Roche | MDRD | MACE |
|
| |||||||||||||
| Bosselman, 2013 13 | Outpt | HF | Cohort | 1 | 415 | 252 (61) | 71 | 72 | Mod | NR | Roche | MDRD | Mortality |
|
| |||||||||||||
| Bruch, 2008 14 | Outpt | HF | Cohort | 1 | 341 | 57 (17) | 79 | 57 | Mod | ESRD | Roche | MDRD | Card. death |
|
| |||||||||||||
| Chrysochou, 2009 15 | Outpt | RAS | Cohort | 1 | 82 | 31 (38) | 62 | 72 | Mod | NR | Roche | CG | Mortality |
|
| |||||||||||||
| DeFilippi, 2007 5 | ER | SOB | Cohort | 1 | 831 | 224 (27) | 46 | 66 | Good | None | Roche | MDRD | Mortality |
|
| |||||||||||||
| Doi, 2011 16,17 | Outpt | Comm | Cohort | N/A | 3104 | 127 (4) | 42 | 61 | Mod | None | Roche | MDRD | MACE |
|
| |||||||||||||
| Fabbian, 2013 18 | Inpt | HF | Cohort | 1 | 134 | 35 (26) | 41 | 79 | Poor | NR | Roche | CKD-Epi | Mortality |
|
| |||||||||||||
| Fu, 2013 6 | Inpt | CAD | Cohort | 1 | 999 | 215 (22) | 91 | 86 | Mod | None | Siemens | MDRD | Mortality |
|
| |||||||||||||
| Gardner, 2007 19 | Outpt | HF | Cohort | 1 | 182 | 40 (22) | 80 | 51 | Mod | None | Roche | MDRD | Mortality |
|
| |||||||||||||
| Horii, 2013 20 | Outpt | CAD | Cohort | 1 | 356 | 132 (37) | 71 | 65 | Mod | NR | Shionogi | MDRD | Mortality |
|
| |||||||||||||
| James, 2003 21,22 | Inpt | ACS | RCT | 458 | 7800 | 553 (7) | 62 | 65 | Good | ESRD, sCr>5 | Roche | CG | Mortality |
|
| |||||||||||||
| Lassus, 2007 23 | Inpt | HF | Cohort | 14 | 480 | 122 (25) | 50 | 75 | Mod | None | Roche | Cystatin C | Mortality |
|
| |||||||||||||
| Lazzeri, 2012 24 | Inpt | ACS | Cohort | 1 | 646 | 75 (12) | NR | NR | Poor | NR | Roche | CKD-Epi | Mortality |
|
| |||||||||||||
| Leuchte, 2007 25 | Outpt | PHTN | Cohort | 1 | 118 | 14 (12) | 37 | 54 | Mod | AKI, ESRD | Roche | Rule | Mortality |
|
| |||||||||||||
| Masson, 2006 26,27 | Outpt | HF | RCT | 302 | 3916 | 758 (19) | 80 | 62 | Good | sCr>2.5 | Roche | eGFR | Mortality |
|
| |||||||||||||
| Mueller, 2009 28,29 | Inpt | PAD | Cohort | 1 | 487 | 114 (23) | 70 | 70 | Mod | None | Roche | MDRD | Mortality |
|
| |||||||||||||
| Ndrepepa, 2007 30,31 | Inpt | CAD | Cohort | 1 | 1552 | 171 (11) | 76 | 67 | Mod | sCr>2 | Roche | sCr | Mortality |
|
| |||||||||||||
| Okkonen, 2011 32,33 | Inpt | ICU | Cohort | 25 | 602 | 174 (29) | 66 | 60 | Good | None | Roche | Renal SOFA | Mortality |
|
| |||||||||||||
| Palmer, 2009 34 | Inpt | ACS | Cohort | 1 | 1063 | 277 (26) | 79 | 62 | Mod | None | IA | MDRD | Mortality |
|
| |||||||||||||
| Petretta, 2007 35 | Inpt | HF | Cohort | 1 | 153 | 32 (21) | 64 | 72 | Mod | ESRD | Roche | CG | Mortality |
|
| |||||||||||||
| Pimenta, 2007 36 | Inpt | HF | Cohort | 1 | 283 | 125 (44) | 48 | 73 | Mod | ESRD | Roche | MDRD | Mortality |
|
| |||||||||||||
| Ruan, 2014 37 | Inpt | HF | Cohort | 1 | 162 | 45 (28) | 53 | 52 | Poor | NR | IA | Cystatin C | Mortality |
|
| |||||||||||||
| Scheven, 2012 38 | Outpt | Comm | Cohort | N/A | 1505 | 583 (39) | 50 | 50 | Mod | None | Roche | CKD-Epi | MACE |
|
| |||||||||||||
| Scrutinio, 2014 39 | Inpt | HF | Cohort | 2 | 908 | 234 (26) | 77 | 66 | Mod | None | NR | eGFR | Mortality |
|
| |||||||||||||
| Van Kimmenade, 2006 40,41 | ER | SOB | Cohort | 4 | 720 | 89 (12) | 51 | 75 | Good | Varied | Roche | MDRD | Mortality |
|
| |||||||||||||
| Von Haehling, 2007 42 | Outpt | HF | Cohort | 5 | 774 | 239 (31) | 92 | 62 | Poor | NR | Roche | sCr | Mortality |
|
| |||||||||||||
| Waldum, 2013 43 | Outpt | HF | Cohort | 13 | 2076 | 370 (18) | 74 | 69 | Good | None | Varied | MDRD | Mortality |
ER, emergency room; Outpt, outpatient; Inpt, inpatient; HF, congestive heart failure; CAD, coronary artery disease; ACS, acute coronary syndrome; Comm, Community; RAS, renal artery stenosis; CKD, chronic kidney disease; SOB, shortness of breath; PAD, peripheral arterial disease; PHTN, Pulmonary Hypertension; RCT, randomized controlled trial; ESRD, end-stage renal disease; IA, immunoassay; MDRD, Modified Diet in Renal Disease; sCr, serum creatinine (mg/dl); CG, Cockcroft-Gault; eGFR, estimated GFR (ml/min/1.73m2); SOFA, sequential organ failure assessment score; MACE, major adverse cardiac events; Mod, moderate; NR, not reported
References located in Online Supplementary material
Quality score assessed by Hayden criteria17
Table 2B. Summary Data for Prognosis*.
| Author, Year | Renal Function Categories | # | # Events (%) | AUC | Cut-point (pg/ml) | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Receiver Operator Analysis | |||||||
| Bruch, 2008 | eGFR>=60 | NR | NR | 0.81 (SE 0.04) | 1474 | NR | NR |
|
| |||||||
| <60 | NR | NR | 0.81 (SE 0.04) | 1474 | NR | NR | |
|
| |||||||
| Fu, 2013 | eGFR>=60 | 641 | 105 | 0.75 (CI 0.70-0.81) | 370 | 0.77 | 0.63 |
|
| |||||||
| <60 | 358 | 110 | 0.82 (CI 0.77-0.86) | 2584 | 0.65 | 0.84 | |
|
| |||||||
| Horii, 2013 | eGFR>30 | 1078 | 104 | 0.74 | 259 | 0.77 | 0.59 |
|
| |||||||
| <30 | 113 | 28 | 0.76 | 5809 | 0.79 | 0.63 | |
|
| |||||||
| Masson, 2006 | eGFR>=60 | 2012 | 295 | 0.65 (SE 0.02) | 769 | 0.64 | 0.59 |
|
| |||||||
| <60 | 1660 | 407 | 0.68 (SE 0.02) | 2023 | 0.55 | 0.72 | |
|
| |||||||
| Ndrepepa, 2007 | sCr<=1.3 | 1318 | 125 | 0.73 (CI 0.69-0.78) | 546 | 0.67 | 0.67 |
|
| |||||||
| >1.3 | 234 | 46 | 0.81 (CI 0.75-0.88) | 1699 | 0.76 | 0.76 | |
|
| |||||||
| Okkonen, 2011 | w/ cardiac history | ||||||
| sCr<1.2 | NR | NR | 0.77 (CI 0.67-0.84) | 1947 | NR | NR | |
|
| |||||||
| 1.2-1.9 | NR | NR | 0.35 (CI 0.40-0.77) | 3961 | NR | NR | |
| >1.9 | NR | NR | 0.66 (CI 0.38-0.69) | 11405 | NR | NR | |
|
| |||||||
| w/o cardiac history | |||||||
| sCr<1.2 | NR | NR | 0.71 (CI 0.63-0.79) | 417 | NR | NR | |
|
| |||||||
| 1.2-1.9 | NR | NR | 0.53 (CI 0.31-0.75) | 2991 | NR | NR | |
| >1.9 | NR | NR | 0.69 (CI 0.54-0.84) | 6606 | NR | NR | |
|
| |||||||
| Author, Year | Renal Function Categories | # | # Events (%) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) † | ||
| Measures of Association | |||||||
|
| |||||||
| Alehagen, 2009 | CysC<1.42 | 229 | 62 (27) | -- | -- | ||
|
| |||||||
| >1.42 | 235 | 76 (32) | -- | -- | |||
|
| |||||||
| Anwaruddin, 2006 | eGFR>60 | 392 | 13 (3) | -- | -- | ||
|
| |||||||
| <60 | 207 | 17 (8) | -- | 1.61 (1.14-2.26) | |||
|
| |||||||
| Astor, 2008 | eGFR>=45 | 558 | 72 (13) | -- | 1.38 (1.15-1.66) | ||
|
| |||||||
| <45 | 436 | 62 (14) | -- | 1.50 (1.24-1.83) | |||
|
| |||||||
| Chrysochou, 2009 | eGFR>=30 | 46 | NR | -- | Low NTproBNP: Ref, High 5.48(0.45-67.36) | ||
|
| |||||||
| <30 | 47 | NR | -- | Low: 3.63 (0.17-79.44), High 8.30 (0.97-74.96) | |||
|
| |||||||
| DeFillipi, 2007 | eGFR>=60 | 438 | 83 (19) | -- | -- | ||
|
| |||||||
| <60 | 393 | 143 (36) | -- | -- | |||
|
| |||||||
| Doi, 2011 | eGFR>=60 | 2239 | 58 (3) | -- | 1.61 (1.16-2.24) | ||
|
| |||||||
| <60 | 807 | 69 (9) | -- | 1.58 (1.31-1.91) | |||
|
| |||||||
| Fabbian, 2013 | eGFR>=60 | 42 | 5 (12) | -- | 1.16 (0.19-7.03) | ||
|
| |||||||
| <60 | 77 | 30 (39) | -- | 2.89 (1.00-8.33) | |||
|
| |||||||
| Fu, 2013 | eGFR>=60 | 641 | 105 (16) | 1.69 (1.50-1.90) | 1.38 (1.19-1.61) | ||
|
| |||||||
| <60 | 358 | 110 (31) | 1.77 (1.57-1.98) | 1.54 (1.32-1.80) | |||
|
| |||||||
| James, 2003 | eGFR>=66 | NR | 119 | 3.10 (2.05-4.70)‡ | -- | ||
|
| |||||||
| <66 | NR | 434 | 5.48 (4.24-7.08)‡ | -- | |||
|
| |||||||
| Lassus, 2007 | CysC<1.13 | 159 | 15 (9) | -- | -- | ||
|
| |||||||
| 1.13-1.55 | 159 | 35 (22) | -- | -- | |||
| >1.55 | 159 | 61 (38) | -- | -- | |||
|
| |||||||
| Lazzeri, 2012 | eGFR>=60 | 517 | 42 (8) | -- | 1.005 (1.001-1.010)§ ‖ | ||
|
| |||||||
| <60 | 129 | 33 (26) | -- | 1.003 (1.001-1.005)§ ‖ | |||
|
| |||||||
| Mueller, 2009 | eGFR>=74 | 244 | 55 (23) | 4.20 (2.52-6.95)‡ | -- | ||
|
| |||||||
| <74 | 243 | 59 (24) | 4.75 (2.13-10.60)‡ | -- | |||
|
| |||||||
| Palmer, 2009 | eGFR>=60 | 889 | 205 (23) | 2.50 (1.94-3.24)§ | -- | ||
|
| |||||||
| <60 | 149 | 72 (48) | 2.29 (1.40-3.76)§ | -- | |||
|
| |||||||
| Pimenta, 2007 | eGFR>=90 | 164 | 61 (37) | -- | 1.64 (0.98-2.76) | ||
|
| |||||||
| <90 | 119 | 60 (50) | -- | 2.53 (1.27-5.03) | |||
|
| |||||||
| Ruan, 2014 | CysC<1.11 | 42 | 4 (10) | -- | -- | ||
|
| |||||||
| 1.11-1.46 | 42 | 11 (26) | -- | -- | |||
| >1.46 | 42 | 26 (62) | -- | -- | |||
|
| |||||||
| Scheven, 2012 | eGFR>=60 | NR | NR | -- | 1.49 (1.10-2.00) | ||
|
| |||||||
| <60 | NR | NR | -- | 1.55 (1.10-2.20) | |||
|
| |||||||
| Scrutinio, 2014 | eGFR>=60 | 409 | 53 (13) | -- | 2.09 (1.14-3.85) | ||
|
| |||||||
| 30-59 | 399 | 126 (32) | -- | 1.70 (1.17-2.49) | |||
| <30 | 99 | 55 (56) | -- | 3.33 (1.33-8.33) | |||
|
| |||||||
| Von Haehling, 2007 | sCr<1 | NR | NR | -- | 2.65 (1.98-3.55) | ||
|
| |||||||
| 1-1.3 | NR | NR | -- | 1.80 (1.47-2.20) | |||
| r>1.3 | NR | NR | -- | 1.30 (1.19-1.42) | |||
|
| |||||||
| Waldum, 2013 | eGFR>=60 | 886 | NR | -- | 1.92 (1.36-2.71) | ||
|
| |||||||
| <60 | 508 | NR | -- | 1.57 (0.92-2.68) | |||
eGFR, estimated GFR (1.73/ml/m2); sCr, serum creatinine (mg/dl); NR, not reported
Data provided only for studies that presented quantitative data.
Reference group for patients with preserved renal function is patients with preserved renal function and low NT-proBNP; reference group for patients with renal dysfunction is patients with renal dysfunction and low NT-proBNP.
Relative Risk.
Odds Ratio.
Per 1 unit increase.
Correlation of GFR and NT-proBNP Levels
Eleven studies reported correlation coefficients between estimated GFR (eGFR) and NT-proBNP, 21,22,24,26-33. The correlation coefficients were statistically significant and ranged from -0.21 to -0.58. A sub-set of studies reported NT-proBNP levels by patients' eGFR; NT-proBNP levels consistently increase as renal function declines (Online Table 2).
Diagnostic Ability of NT-proBNP
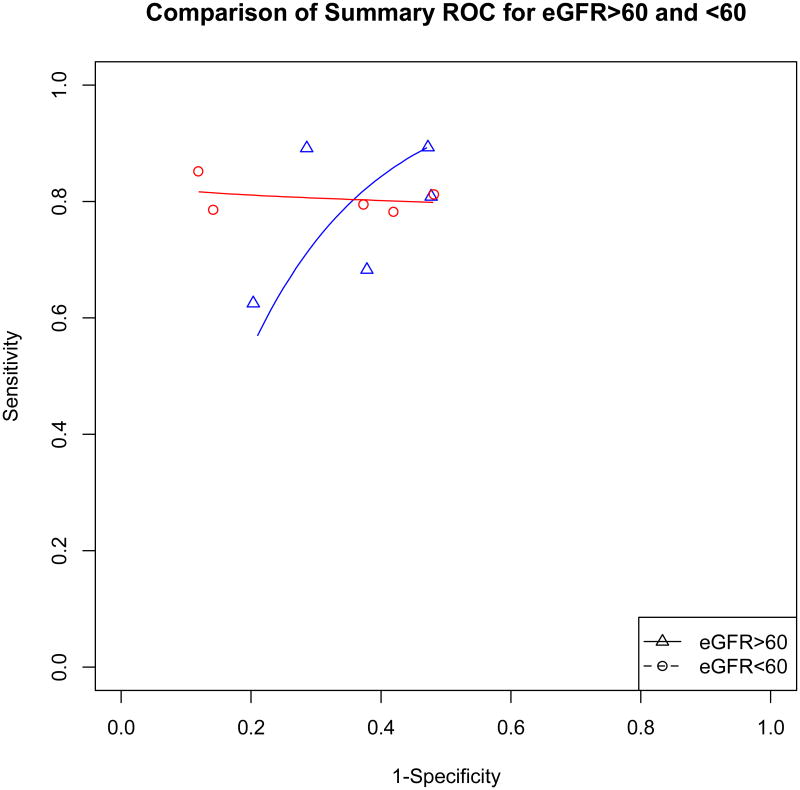
For diagnosing ADHF, the cut-points in patients with an eGFR<60 ml/min/1.73 m2 were roughly two-fold higher than the cut-points in patients with an eGFR>60ml/min/1.73 m2 (Table 1B). Even with higher cut-points, the specificity and sensitivity were often slightly lower in patients with eGFR<60 compared to patients with eGFR >60 (Figure 1A). While we were unable to generate confidence intervals for the AUC to evaluate if the summary ROCs are significantly different, the curves overlap on visual inspection (Figure 1B).
Figure 1A. Forest Plots for Sensitivity and Specificity for Diagnosis of ADHF.
Figure 1B. Comparison of Summary ROC for eGFR>60 and <60.
Data points represent the overall sensitivity and 1-specificity from an individual study.
Summary ROC curve was generated by the Moses method.
Prognostic Ability of NT-proBNP
Twelve studies provided crude estimates for the association between NT-proBNP and mortality, either in the form of event rates, relative risk or sensitivity/specificity data. When comparing patients with preserved renal function and elevated NT-proBNP to patients with preserved renal function and normal NT-proBNP, the unadjusted pooled risk ratio (RR) was 3.01 (95% CI, 2.53-3.58) (Figure 2A). Correspondingly, the pooled risk ratio for patients with renal dysfunction was 3.25 (95% CI, 2.45-4.30) although there was a higher event rate (20.9%) than patients with preserved renal function (11.9%) (Figure 2B). The pooled risk ratios between patients with preserved and diminished renal function were not significantly different (p-value=0.652), and there was significant heterogeneity in both sub-groups (Figure 2A and 2B). Four of the studies used higher NT-proBNP cut-points for patients with renal dysfunction 28,33-35, while the remaining studies used the same NT-proBNP cut-points in the different sub-groups of renal function.
Figure 2A. Crude Estimates of Mortality in Patients with Preserved Renal Function.
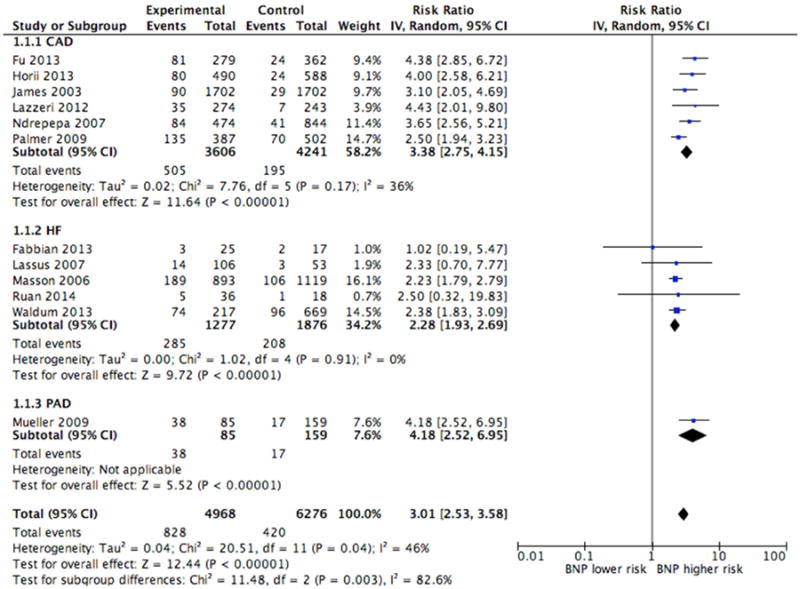
CAD=coronary artery disease, HF=heart failure, PAD=peripheral artery disease
Figure 2B. Crude Estimates of Mortality in Patients with Diminished Renal Function.
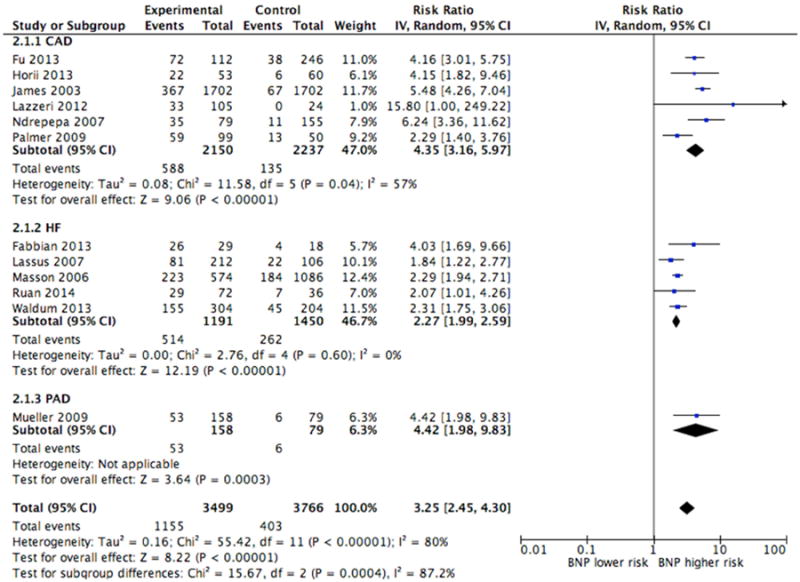
CAD=coronary artery disease, HF=heart failure, PAD=peripheral artery disease
Nine studies provided adjusted estimates for the association between NT-proBNP and mortality. While the studies adjusted for different variables, the pooled risk ratios for patients with preserved and diminished renal function were similar, RR 1.43 (95% CI, 1.34-1.53) and RR 1.59 (95% CI, 1.41-1.80) respectively (Figure 3A and 3B), and there was no statistically significant difference between the two groups (p-value=0.141). There was significant heterogeneity in patients with renal dysfunction, which resolved after removing the study by Waldum 36 (Online Figure 2). The authors noted in correspondence that these data did not meet the proportional hazards assumption.
Figure 3A. Adjusted Estimates of Mortality in Patients with Preserved Renal Function.

Figure 3B. Adjusted Estimates of Mortality in Patients with Diminished Renal Function.

Assessment of Bias
Funnel plots were created to assess publication bias (Online Figure 3). Funnel plots for crude effect estimates demonstrated heterogeneity but were not markedly asymmetric. There was publication bias favoring small studies with large adjusted effect estimates, especially in patients with renal dysfunction.
Meta-regression for Crude Effect Estimates of NT-proBNP and Mortality
Since there was significant heterogeneity in the crude effect estimates for NT-proBNP and mortality, we undertook meta-regression. Samples comprised of patients with CAD or HF explained significant proportions of the heterogeneity (Online Table 3). In patients with renal dysfunction, the slope was significantly positive if studies sampled patients with CAD suggesting that elevated NT-proBNP confers a larger magnitude increase in risk of mortality in patients with renal dysfunction and CAD compared to patients with renal dysfunction and other co-morbidities. In patients with preserved or diminished renal function, the slope was significantly negative if studies sampled patients with congestive heart failure suggesting that elevated NT-proBNP confers a smaller magnitude increase in risk of mortality in patients with HF compared to patients with other comorbidities. The type of renal function estimate, presence of different NT-proBNP cut-points for kidney function sub-groups, or NT-proBNP assay failed to explain a significant proportion of the heterogeneity.
Discussion
While patients with renal dysfunction have higher plasma levels of NT-proBNP, this systematic review and meta-analysis demonstrates that NT-proBNP remains useful for diagnosis of ADHF or prognosis in these patients. If higher cut-points are used, NT-proBNP still has acceptable sensitivity and specificity for diagnosis of ADHF in patients with renal dysfunction. Furthermore, elevated NT-proBNP in patients with renal dysfunction compared to patients with normal NT-proBNP and renal dysfunction confers similarly increased risk of mortality as in patients with preserved renal function. This relationship was consistent among different patient care settings, although meta-regression found that the relationship between NT-proBNP and mortality varied depending upon the patients' comorbidities.
Diagnosis of ADHF
Among the studies identified addressing the diagnostic ability of NT-proBNP for ADHF, the cut-points varied from 1200 pg/ml to >6,000 pg/ml for patients with an eGFR<60 ml/min/1.73m2, all of which are higher than the standard “Januzzi cut-points”. Despite the higher cut-points, the AUC, sensitivity and specificity in patients with renal dysfunction was often slightly lower, although the summary ROC curves for patients with preserved and diminished renal function overlap on visual inspection. Higher NT-proBNP cut-points did not always result in a higher sensitivity and lower specificity; this may be because the “gold standard” was different in each study. Furthermore, the “gold standard” often partially entailed chart review by a clinician, which relies heavily on an individual's clinical judgment and likely varied between studies. Thus, while it appears that NT-proBNP retains diagnostic utility if using higher cut-points in patients with renal dysfunction, it is possible that more patients would be falsely identified with ADHF in clinical practice. We would encourage prospective clinical studies be completed specifically for patients with renal dysfunction to help identify more precise cut-points.
Prognosis
Regarding prognosis, the crude and adjusted pooled RRs for NT-proBNP were not significantly different between patients with preserved and diminished renal function, although the absolute event rates were two-fold higher in patients with diminished renal function. This suggests that among patients with renal dysfunction, elevated NT-proBNP confers the same increased risk of mortality as compared to elevated NT-proBNP in patients without renal dysfunction. However, since patients with renal dysfunction are overall at higher risk of mortality, the absolute mortality rates are higher than patients without renal dysfunction. Thus, elevated NT-proBNP levels in patients with renal dysfunction are still capturing important prognostic information. Since it is uncertain if NT-proBNP is completely renally excreted, markedly elevated levels of NT-proBNP in patients with diminished renal function could be due to either decreased clearance or increased cardiac production. Both of these factors could contribute to increased mortality.
There was significant heterogeneity for the pooled unadjusted risk ratios, while the adjusted risk ratios did not have significant heterogeneity after excluding one study. Underlying patient population co-morbidities were a significant contributor to heterogeneity in meta-regression, which suggests that the prognostic value of NT-proBNP may vary depending upon the patient's clinical diagnosis. It seems that elevated NT-proBNP confers a larger magnitude increase in mortality in patients with renal dysfunction and CAD compared to elevated NT-proBNP in patients with HF. This is likely because elevated NT-proBNP in patients with CAD provides new information that the patient additionally has ventricular stress, while elevated NT-proBNP in patients with HF does not provide new information regarding the presence of ventricular stress. It was reassuring that the type of renal function estimate, NT-proBNP assay or different NT-proBNP cut-points for kidney function sub-groups did not contribute significantly to the observed heterogeneity.
As the studies in this systematic review demonstrate, NT-proBNP levels are often markedly elevated in patients with renal dysfunction, which presents a conundrum in clinical practice. Is this cardiac biomarker elevated because of diminished renal clearance or because of extra-renal pathophysiology? The specific mechanisms for clearance of NT-proBNP are not fully elucidated, so these results could be due to either decreased clearance or increased cardiac production. It is plausible that these results are due to increased cardiac production since the anemia, uremia and secondary hyperparathyroidism from chronic kidney disease is known to have myriad negative ramifications on myocardium 37-39.
Limitations
This meta-analysis has several limitations. First, the studies varied regarding NT-proBNP assay, NT-proBNP cut-points, renal function measurements and how patients were divided into subgroups. This could introduce heterogeneity into our study, although this was not detected by meta-regression techniques. Second, there were few studies regarding diagnosis of ADHF and hence, we were unable to analyze the data with statistically rigorous methodology to determine if there was a statistically significant difference between the summary ROCs. Third, we did not have patient-level data and were unable to identify optimal cut-points for NT-proBNP in diagnostic and prognostic scenarios. Fourth, there were few patients with end-stage renal disease in these studies, so these results should not be extrapolated to the dialysis population. Fifth, many patients with HF have dynamic renal function, thus the NT-proBNP cut-points in the diminished renal function sub-groups may not be precise.
Conclusions
In conclusion, this meta-analysis demonstrates that assessment of NT-proBNP levels in patients with preserved and diminished renal function provides useful prognostic information regarding mortality and would likely have diagnostic utility if cut-points were identified for various GFR categories. Future studies should explore establishing accepted diagnostic cut-points for ADHF in patients with diminished GFR. While markedly elevated NT-proBNP in a patient with renal dysfunction may be partially due to decreased clearance, it still portends a higher absolute risk for mortality compared to patients with normal renal function. It would be beneficial to elucidate how natriuretic peptide processing is altered in patients with diminished renal function. There is a relative dearth of information regarding the test characteristics of novel biomarkers in patients with renal dysfunction. Investigators should make every effort to sample a sufficient number of patients with renal disease and investigate the test characteristics separately in this important and vulnerable group of patients.
Supplementary Material
Figure 1. Flow Diagram of Studies Considered for Inclusion
Figure 2. Adjusted Estimates of Mortality in Patients with Preserved and Diminished Renal Function (without Waldum)
Figure 3. Funnel Plots for Prognosis
Table 1. Search Methods in Ovid (Medline for Prognosis)
Table 2A. Reported NT-proBNP Levels in Patients with eGFR>60 and eGFR<60
Table 2B. Reported NT-proBNP Levels by eGFR
Table 3. Meta-regression for Crude Estimates of NT-proBNP and Mortality
References. References of Studies included in Systematic Review and Meta-analysis
Clinical Perspectives.
Competency in Medical Knowledge
NT-proBNP levels are often elevated in patients with renal dysfunction, which may complicate interpretation for diagnosis of ADHF or prognosis. For diagnosis of ADHF, NT-proBNP seems to have similar diagnostic properties in patients with renal dysfunction compared to patients without renal dysfunction provided higher cut-offs are used. While patients with renal dysfunction have an absolute mortality rate that is approximately double that of patients without renal dysfunction, NT-proBNP still captures useful prognostic information.
Translational Outlook
NT-proBNP levels are commonly elevated in patients with renal dysfunction, although it is not clear if this is due to increased cardiac production or decreased renal clearance. Future studies should clarify the biology of NT-proBNP production and clearance, especially in patients with renal dysfunction. Investigators should make a concerted effort to separately analyze biomarker characteristics in patients with renal dysfunction.
Acknowledgments
We thank Drs Fabbian, Lu, Darmon, Waldum and Masson (on behalf of the Val-HeFT trial) who kindly provided further data for the meta-analysis.
Grants: JAS is supported by NIH (T32DK007276-36), JMT is supported by NIH (K23HL114868 and L30HL115790), and CRP is supported by the NIH (K24DK090203).
Abbreviations List
- NT-proBNP
Plasma Amino Amino-terminal Pro B-type Natriuretic Peptide
- ADHF
acute decompensated heart failure
- HF
heart failure
- CAD
coronary artery disease
- ROC
receiver operator curve
Footnotes
Disclosures: The authors do not have any relationships with industry to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartmann F, Packer M, Coats AJ, et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110(13):1780–1786. doi: 10.1161/01.CIR.0000143059.68996.A7. [DOI] [PubMed] [Google Scholar]
- 2.Kragelund C, Grønning B, Køber L, Hildebrandt P, Steffensen R. N-Terminal Pro–B-Type Natriuretic Peptide and Long-Term Mortality in Stable Coronary Heart Disease. New England Journal of Medicine. 2005;352(7):666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 3.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 4.Laramee P, Wonderling D, Swain S, Al-Mohammad A, Mant J. Cost-effectiveness analysis of serial measurement of circulating natriuretic peptide concentration in chronic heart failure. Heart. 2013;99(4):267–271. doi: 10.1136/heartjnl-2012-302692. [DOI] [PubMed] [Google Scholar]
- 5.Moe GW, Howlett J, Januzzi JL, Zowall H Canadian Multicenter Improved Management of Patients With Congestive Heart Failure Study I. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103–3110. doi: 10.1161/CIRCULATIONAHA.106.666255. [DOI] [PubMed] [Google Scholar]
- 6.Pfisterer M, Buser P, Rickli H, et al. BNP-guided vs symptom-guided heart failure therapy the trial of intensified vs standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. JAMA - Journal of the American Medical Association. 2009;301(4):383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL., Jr Biology of the natriuretic peptides. The American journal of cardiology. 2008;101(3a):3–8. doi: 10.1016/j.amjcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Huntley BK, Sandberg SM, Heublein DM, Sangaralingham SJ, Burnett JC, Jr, Ichiki T. Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure. Circulation Heart failure. 2015;8(1):89–97. doi: 10.1161/CIRCHEARTFAILURE.114.001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenov AG, Tamm NN, Seferian KR, et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem. 2010;56(7):1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 10.Vodovar N, Seronde MF, Laribi S, et al. Post-translational modifications enhance NT-proBNP and BNP production in acute decompensated heart failure. European heart journal. 2014;35(48):3434–3441. doi: 10.1093/eurheartj/ehu314. [DOI] [PubMed] [Google Scholar]
- 11.van Kimmenade RR, Januzzi JL, Jr, Bakker JA, et al. Renal clearance of B-type natriuretic peptide and amino terminal pro-B-type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol. 2009;53(10):884–890. doi: 10.1016/j.jacc.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Miller WL, Phelps MA, Wood CM, et al. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circulation Heart failure. 2011;4(3):355–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260. [DOI] [PubMed] [Google Scholar]
- 13.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13(6):422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47(10):1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC medical research methodology. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden JA, Côté P, Bombardier C. Evaluation of the Quality of Prognosis Studies in Systematic Reviews. Annals of Internal Medicine. 2006;144(6):427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 18.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Statistics in medicine. 1993;12(14):1293–1316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 19.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 20.Doebler P, Holling H. Meta-analysis of Diagnostic Accuracy with mada. 2013 doi: 10.1007/s11336-014-9430-0. [DOI] [PubMed] [Google Scholar]
- 21.Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. Journal of the American College of Cardiology. 2006;47(1):91–97. doi: 10.1016/j.jacc.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 22.Chenevier-Gobeaux C, Claessens YE, Voyer S, Desmoulins D, Ekindjian OG. Influence of renal function on N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients admitted for dyspnoea in the Emergency Department: comparison with brain natriuretic peptide (BNP) Clinica chimica acta; international journal of clinical chemistry. 2005;361(1-2):167–175. doi: 10.1016/j.cccn.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Colak A, Cuhadar S, Golcuk B, Golcuk Y, Ozdogan O, Coker I. Effect of renal failure on N-terminal Pro-Brain natriuretic peptide in patients admitted to emergency department with acute dyspnea. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 2014;14(6):519–524. doi: 10.5152/akd.2014.4944. [DOI] [PubMed] [Google Scholar]
- 24.DeFilippi CR, Seliger SL, Maynard S, Christenson RH. Impact of renal disease on natriuretic peptide testing for diagnosing decompensated heart failure and predicting mortality. Clinical Chemistry. 2007;53(8):1511–1519. doi: 10.1373/clinchem.2006.084533. [DOI] [PubMed] [Google Scholar]
- 25.Gorissen C, Baumgarten R, De Groot M, Van Haren E, Kragten H, Leers M. Analytical and clinical performance of three natriuretic peptide tests in the emergency room. Clinical Chemistry and Laboratory Medicine. 2007;45(5):678–684. doi: 10.1515/CCLM.2007.119. [DOI] [PubMed] [Google Scholar]
- 26.Astor BC, Yi S, Hiremath L, et al. N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: The African American Study of Kidney Disease and Hypertension (AASK) Circulation. 2008;117(13):1685–1692. doi: 10.1161/CIRCULATIONAHA.107.724187. [DOI] [PubMed] [Google Scholar]
- 27.Coquet I, Darmon M, Doise JM, et al. Performance of N-terminal-pro-B-type natriuretic peptide in critically ill patients: A prospective observational cohort study. Critical Care. 2008;12(6) doi: 10.1186/cc7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu S, Luo L, Ye P, et al. The ability of NT-proBNP to detect chronic heart failure and predict all-cause mortality is higher in elderly Chinese coronary artery disease patients with chronic kidney disease. Clinical Interventions in Aging. 2013;8:409–417. doi: 10.2147/CIA.S42700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazzeri C, Valente S, Chiostri M, Attana P, Picariello C, Gensini GF. The influence of renal function on the prognostic value of Nt-pro brain natriuretic peptide in St-elevation myocardial infarction. International Journal of Cardiology. 2012;156(3):333–335. doi: 10.1016/j.ijcard.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Leuchte HH, El Nounou M, Tuerpe JC, et al. N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest. 2007;131(2):402–409. doi: 10.1378/chest.06-1758. [DOI] [PubMed] [Google Scholar]
- 31.Park HJ, Baek SH, Jang SW, et al. Direct comparison of B-type natriuretic peptide and N-terminal pro-BNP for assessment of cardiac function in a large population of symptomatic patients. International Journal of Cardiology. 2010;140(3):336–343. doi: 10.1016/j.ijcard.2008.11.107. [DOI] [PubMed] [Google Scholar]
- 32.van Kimmenade RR, Januzzi JL, Jr, Baggish AL, et al. Amino-terminal pro-brain natriuretic Peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? Journal of the American College of Cardiology. 2006;48(8):1621–1627. doi: 10.1016/j.jacc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 33.Horii M, Matsumoto T, Uemura S, et al. Prognostic value of B-type natriuretic peptide and its amino-terminal proBNP fragment for cardiovascular events with stratification by renal function. Journal of Cardiology. 2013;61(6):410–416. doi: 10.1016/j.jjcc.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Masson S, Latini R, Anand IS, et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: The valsartan heart failure (Val-HeFT) data. Clinical Chemistry. 2006;52(8):1528–1538. doi: 10.1373/clinchem.2006.069575. [DOI] [PubMed] [Google Scholar]
- 35.Ndrepepa G, Braun S, Kastrati A, Schomig A. Area under ROC curve, sensitivity, specificity of N-terminal probrain natriuretic peptide in predicting mortality in various subsets of patients with ischemic heart disease [4] Clinical Research in Cardiology. 2007;96(10):763–765. doi: 10.1007/s00392-007-0562-4. [DOI] [PubMed] [Google Scholar]
- 36.Waldum B, Stubnova V, Westheim AS, Omland T, Grundtvig M, Os I. Prognostic utility of B-type natriuretic peptides in patients with heart failure and renal dysfunction. Clinical Kidney Journal. 2013;6(1):55–62. doi: 10.1093/ckj/sfs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. The Journal of clinical investigation. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? European heart journal. 2010;31(14):1771–1779. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 39.London G. Pathophysiology of cardiovascular damage in the early renal population. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16(Suppl 2):3–6. doi: 10.1093/ndt/16.suppl_2.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Flow Diagram of Studies Considered for Inclusion
Figure 2. Adjusted Estimates of Mortality in Patients with Preserved and Diminished Renal Function (without Waldum)
Figure 3. Funnel Plots for Prognosis
Table 1. Search Methods in Ovid (Medline for Prognosis)
Table 2A. Reported NT-proBNP Levels in Patients with eGFR>60 and eGFR<60
Table 2B. Reported NT-proBNP Levels by eGFR
Table 3. Meta-regression for Crude Estimates of NT-proBNP and Mortality
References. References of Studies included in Systematic Review and Meta-analysis