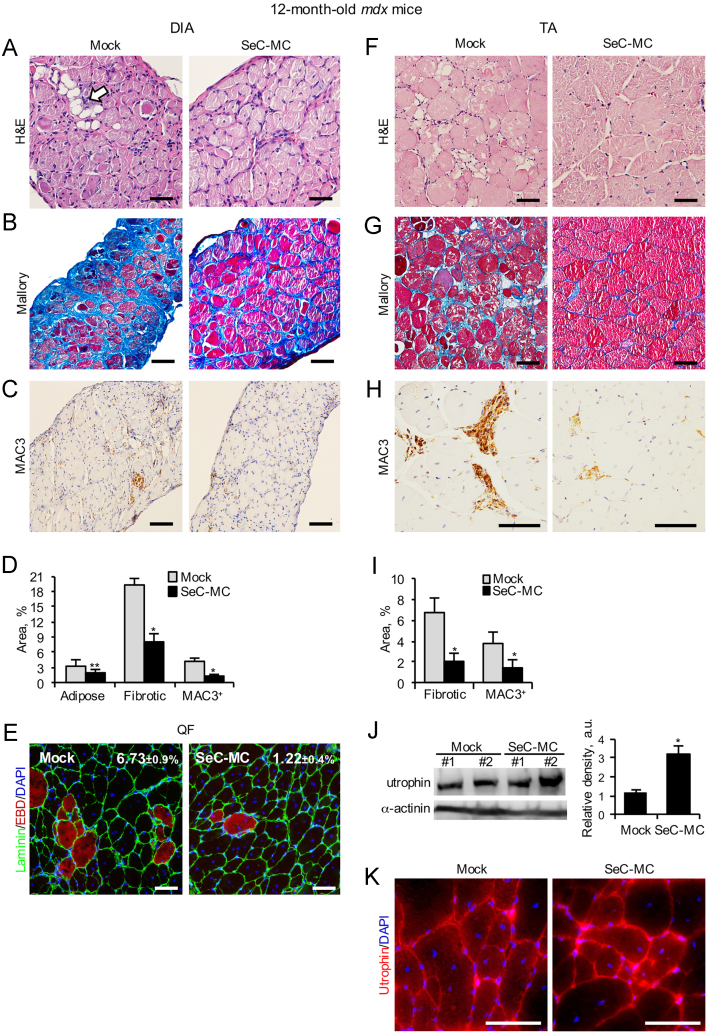
Fig. 1.
SeC-MC ameliorate muscle morphology when i.p. injected into chronic dystrophic mice. (A–D) DIA from 12-month-old mdx mice i.p. injected with SeC-MC (n=6) or empty microcapsules (Mock) (n=6) were analyzed three weeks after injection. H&E (A) and Mallory (B) staining were used to reveal adipose tissue (arrow in A) and fibrotic tissue (blue) infiltrate, respectively, and MAC3 immunohistochemistry (C) was used to detect macrophages in DIA from SeC-MC-treated and mock-treated mice. The average percentages (±SEM) of adipose tissue infiltrate, and fibrotic and MAC3+ areas in mock- and SeC-MC-treated DIA were determined (D). (E) Mdx mice treated as above were injected with Evans Blue Dye (EBD) 24 h before being sacrificed and QF muscles were isolated, cryosectioned and analyzed for detection of EBD infiltration (red). Individual myofibers were delineated with laminin staining (green), and nuclei were counterstained with DAPI (blue). Shown are representative images. Reported are the average percentages (±SEM) of EBD-positive myofibers in each group. (F–K) TA muscles isolated from mice in (A–D) were analyzed by H&E (F) or Mallory (G) staining, and MAC3 immunohistochemistry (H). The mean percentages (±SEM) of fibrotic and MAC3+ areas in mock- and SeC-MC-treated mice were determined (I). Utrophin expression and localization were analyzed by Western blotting (J) and immunofluorescence on frozen sections (K), respectively. The average relative densities (±SEM) of utrophin bands with respect to α-actinin bands were determined (J). * and **, significantly different from mock-treated control at p≤0.001 and p≤0.01, respectively. Original magnification (A–C, E–G), 20×; (H and K), 40×.