Table 1.
Styryl and bis-styryl fluorophores in solution
| Namea | Chemical Structure | λAbsb, nm | λEm
c, nm (Intensity)d |
|---|---|---|---|
|
d1
(BMB)a |
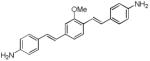
|
408 | 495 (106,800) |
| d2 |
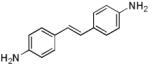
|
362 | 414 (246,900) |
| d3 |
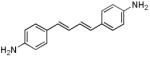
|
383 | 448 (84,300) |
| d4 |
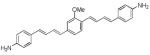
|
400 | 575 (200) |
| d5 |
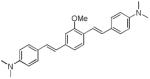
|
415 | 511 (93,300) |
| d6 |
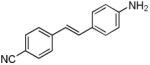
|
385 | 522 (2,800) |
|
d7
(GE3082)a |
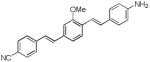
|
417 | 491, 621*
(1,700; 2,520*) |
| d8 |
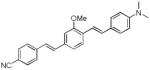
|
412 | 518 (264) |
|
d9
(GE3111)a |
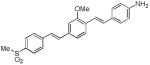
|
412 | 480, 624*
(2,250; 4,700*) |
|
d10
(GE3126)a |
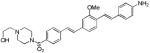
|
414 | 510, 618*
(2,100; 3,970*) |
| d11 |
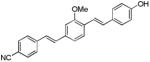
|
396 | 532 (12,640) |
| d12 |
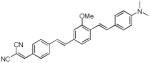
|
467 | 582 (180) |
| d13 |
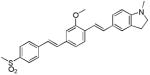
|
419 | 533 (2,900) |
| d14 |
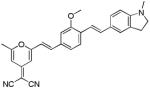
|
482 | 649 (150) |
The d-numbering scheme for each dye is used for simplicity. For previously published fluorophores, the original designations are shown in parenthesis.
Absorbance maximum wavelength measured in DMSO
Emission maximum wavelength measured in DMSO, the relative fluorescence intensity at the maximum wavelength shown in parenthesis
Relative emission intensity values at λEm RFU. For dual emissions, the emission intensities in parentheses correspond to the λEm shown, respectively.
Dual emission (TICT) in polar solvents.
