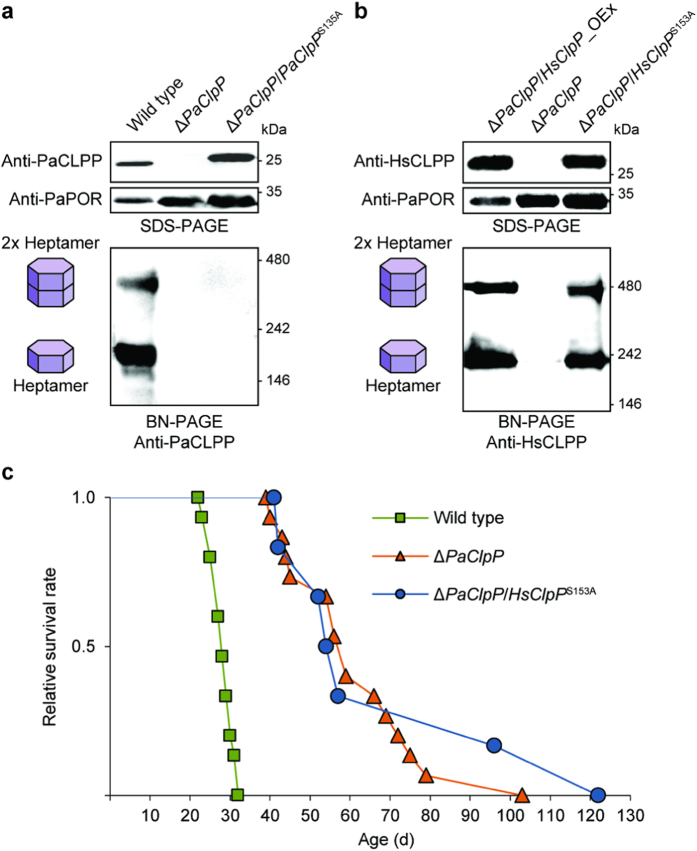
Figure 1. Catalytically inactive human CLPP in P. anserina is suitable for use in a CLPP substrate-trapping assay.
(a) Western blot analyses using a P. anserina CLPP-specific antibody after separation of mitochondrial protein extracts from the indicated strains with SDS-PAGE (above) or BN-PAGE (below). PaPORIN was detected as a loading control. Western blot analysis after SDS-PAGE reveals that monomeric, catalytically inactive PaCLPP (PaCLPPS135A) has an increased size compared to the wild-type PaCLPP monomer (predicted size of mature protein: ~25 kDa). After BN-PAGE, two distinct PaCLPP oligomers, corresponding to the heptameric CLPP ring (at around ~170 kDa) and the full proteolytic chamber formed by two heptamers (at around ~340 kDa), are only visible in the wild-type sample. (b) Western blot analyses as in a, using a Homo sapiens CLPP-specific antibody and mitochondrial protein extracts from the indicated strains. Monomeric, catalytically inactive HsCLPP (HsCLPPS153A) has the same size as the wild-type HsCLPP monomer (predicted size of mature protein: ~24 kDa) and, like wild-type HsCLPP, is able to form heptameric rings (at around ~220 kDa) and the full 14-mer proteolytic chamber (at around ~440 kDa) in P. anserina mitochondria. c, Lifespan of wild type (28.2 ± 0.7; n = 15), ΔPaClpP (61.3 ± 4.3; n = 15; P = 3.4E-06), and ΔPaClpP/HsClpPS153A (70.5 ± 12.8; n = 6; P = 3.7E-05) isolates at 27 °C. Data given in parentheses are mean lifespan ± s.e.m. in days. P-values were determined in comparison to the wild-type sample by two-tailed Wilcoxon rank-sum test.