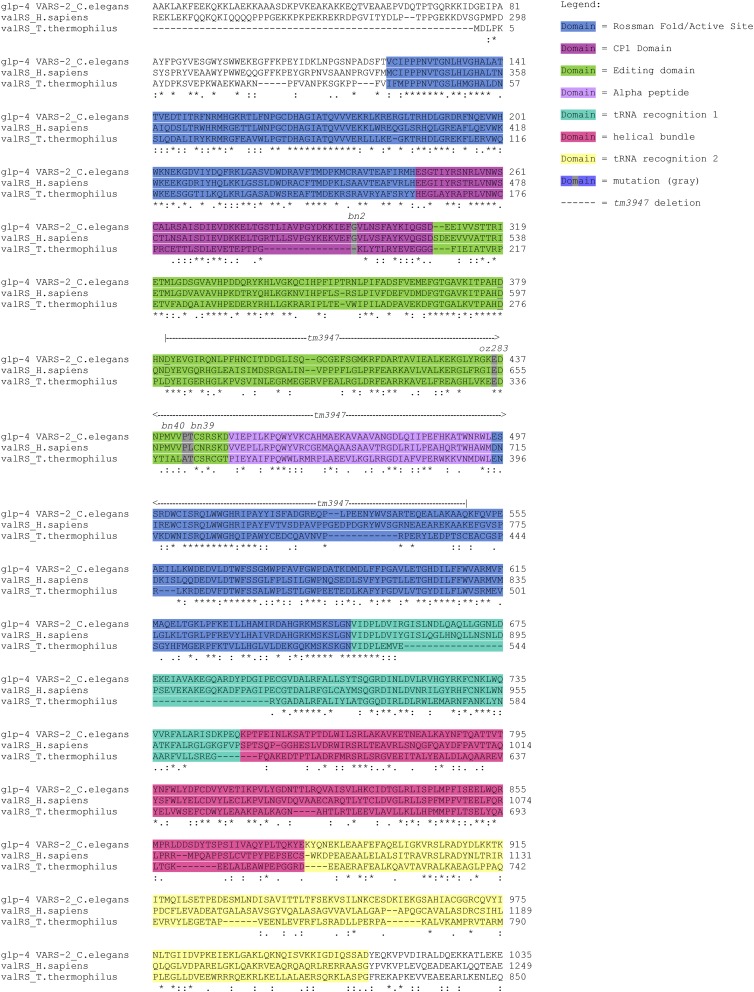
Figure 2.
Alignment of C. elegans glp-4 VARS-2 with human and Thermus thermophilus orthologs. Alignment performed with ClustalW (Larkin et al. 2007). Protein domains are color coded—the split Rossman fold in blue, CP1 domain in magenta, editing domain in green, alpha peptide in purple, transfer RNA (tRNA) recognition domain 1 in turquoise, helical bundle in pink, and tRNA recognition domain in yellow; this color code is used again in Figures 4A to indicate the relevant domains in the 3D structures. Point mutations (bn2, oz283, bn40, and bn39) are marked in the alignment in gray, whereas the deletion allele tm3947 is indicated by the dashed line above the alignment. The editing active site aspartates D379 and D382 are underlined. Below the alignment, residues that are identical (*), strongly similar (:), or weakly similar (.) for all three proteins are indicated. Note that glp-4 VARS-2 and human valRS are more closely related to each other than to T. thermophilus.