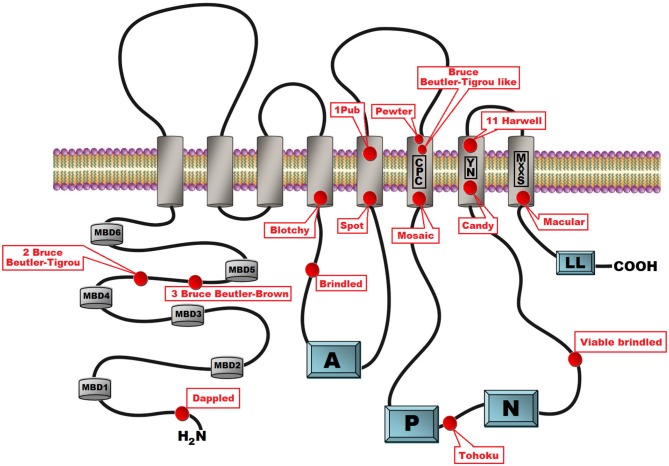
Figure 2.
Schematic presentation of the secondary structure of the ATP7A protein with location of the 15 mottled mutants indicated. ATP7A is a transmembrane protein anchored to the membrane of the Golgi apparatus with eight transmembrane domains. The CPC amino acid motif within the 6th transmembrane domain is assumed to play a direct role in copper ions translocation across the biological membrane. The N-terminal peptide contains six cytoplasmic copper binding domains. Cytoplasmic domains are involved in the catalytic cycle that mediates cupric ions active transport at the cost of ATP hydrolysis. In the catalytic cycle ATP was bind to the nucleotide binding domains (N) and after hydrolysis the γ-phosphate of ATP is transferred to the invariant aspartate residue in the in the phosphorylation domain (P). Energy released by ATP hydrolysis is utilized for ions transport across a membrane. The actuator domain (A) located between the 4th and 5th transmembrane domains plays a key role in the dephosphorylation of the phosphorylated protein. The amino terminal part of the protein contains a dileucine motif (LL) motif that is involved in retrograde transport to the trans Golgi network (TGN).