Abstract
An optical fibre long period grating (LPG) sensor modified with molecularly imprinted polymer nanoparticles (nanoMIPs) for the specific detection of antibiotics is presented. The operation of the sensor is based on the measurement of changes in refractive index induced by the interaction of nanoMIPs deposited onto the cladding of the LPG with free vancomycin (VA). The binding of nanoMIPs to vancomycin was characterised by a binding constant of 4.3±0.1×10−8 M. The lowest concentration of analyte measured by the fibre sensor was 10 nM. In addition, the sensor exhibited selectivity, as much smaller responses were obtained for high concentrations (~ 700 μM) of other commonly prescribed antibiotics such as amoxicillin, bleomycin and gentamicin. In addition, the response of the sensor was characterised in a complex matrix, porcine plasma, spiked with 10 μM of VA.
1. Introduction
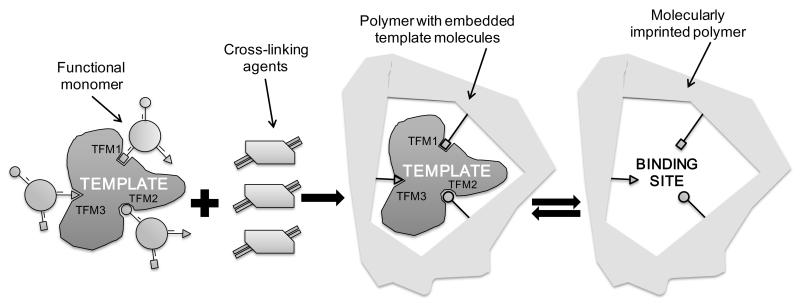
Fibre optic devices coated with functional nanomaterials that exhibit changes in optical properties upon exposure to targeted chemical species have emerged as a promising platform for the development of bio- and chemical sensors1, 2. The characteristics of such sensors, such as sensitivity, selectivity and response/recovery time, depend strongly upon the performance capabilities and properties of the functional layer. Research in the field of fibre optic chemical sensors has focused recently on the development of new functional coatings. The functional materials should be transparent in the appropriate spectral range, change their optical properties under the influence of the specific chemical species, have fast, ideally reversible response with a wide dynamic range, be readily immobilized onto glass/quartz/plastic fibre, and be cheap to manufacture. In this regard, molecularly imprinted polymers (MIPs) provide a universal platform for the creation of artificial receptors that can be well controlled and tailored. Molecular imprinting is one of the most promising approaches for achieving specific molecular recognition. The technique is versatile, as any compound with functional groups can, in principle, be imprinted in different porogens (either water or organic solvents). The basic concept of molecular imprinting is based on the creation of imprints of the template compound (i.e. chemical molecules or biological species that needs to be detected – analytes) in a polymeric matrix, achieved by its incorporation during the polymerization step. Molecular imprinting is achieved in three steps, the first being the complex formation of the template with selected functional monomers, followed by a polymerization step for creation of a matrix that fixes the monomers in the right position to interact with the analyte and with the final step being the removal of the template to leave behind cavities specific for the template, Scheme 1.
Scheme 1.
Schematic illustration of the molecular imprinting procedure3.
Although MIPs have already being identified as stable and low cost mimics of receptors or enzymes for sensing applications4, 5, 6, 7, they are also perceived to have several shortcomings. Among these are the heterogeneous distributions of binding sites, which can produce high levels of non-specific binding and have complex protocols for integration with sensors, which can affect the reproducibility and hence increase the variability between measurements. Some of these issues can be resolved by the use of MIP nanoparticles (nanoMIPs) in standard protocols developed for the immobilisation of antibodies. The particles used here were produced by a solid-phase synthesis approach using a computer-controlled reactor. Such MIP nanoparticles have been shown to have pseudo-monoclonal binding properties, uniform binding sites and high affinity for the target used as the template8, 9. Recently, such MIP nanoparticles were used for the detection of vancomycin in a novel assay based on direct replacement of antibodies10. The assay was capable of measuring vancomycin in buffer and in blood plasma within the range of 0.001–70 nM with a detection limit of 0.0025 nM (2.5 pM).
In this work, nanoparticles produced following the protocol described in [10] were deposited onto an optical fibre long period grating (LPG) with the aim of developing a low cost, highly sensitive and selective vancomycin sensor. Vancomycin is a glycopeptide antiobiotic derived from Amycolatopsis orientalis 11. The mode of action of vancomycin consists of the inhibition of cell wall biosynthesis and alteration of the permeability of the bacterial cell membrane. Vancomycin has been used to treat various serious gram-positive infections. It is a powerful antibiotic, which in high doses can be toxic to the ears and kidneys, whilst at low doses can cause hypersensitivity reactions. Thus it is important to measure accurately the concentration of vancomycin in blood to control its administration to patients. The recommended therapeutic concentration range of vancomycin in plasma is 15-20 mg/L 12. Immunoenzymatic techniques (e.g. fluorescence polarisation immunoassay (FPIA)(TDx), enzyme multiplied immunoassay technique (EMIT), radioimmunoassay (RIA), turbidimetric assays) and chromatographic methods are the most commonly used in clinical environments for the detection of vancomycin in plasma 13. The former, although widely used because of their simplicity and speed, lacks precision, especially for the highest concentrations of the antibiotic14, 15. The latter can be very precise, but the techniques are time consuming and require expensive instruments and highly skilled operators. Therefore there is a need to develop reliable, fast, cheap and easy to use sensors for clinical measurements of vancomycin and antibiotics in general.
Regarding the sensor platform, a long period grating (LPG) is a periodic modulation of the refractive index (RI) of the core of an optical fibre that results in the coupling of light between core and cladding modes. Its combination with nanomaterials provides a prospect for the fabrication of high sensitivity sensors offering specific binding to targeted chemical species16, 17. Recently, we have demonstrated the use of optical fibre LPG sensors modified with different functional coatings for the detection of ammonia18 and aromatic carboxylic acids19.
The coupling between core and cladding modes occurs when the phase matching condition is satisfied:
| (1) |
where λx represents the wavelength at which light is coupled to the LP0x cladding mode, ncore is the effective refractive index of the mode propagating in the core of the fibre, nclad(x) is the effective index of the LP0x cladding mode and ʌ is the period of the LPG.
The transmission spectrum of an optical fibre containing an LPG is characterised by a series of resonance bands centred at the wavelengths satisfying equation 1. The dependences of the cladding modes’ effective refractive indices on the surrounding refractive index are manifested as changes in the central wavelengths of the resonance bands, which has been exploited as a refractive index sensor and non-specific concentration sensor20, and by, coating with the cladding of the optical fibre in the section containing the LPG with a functional material, chemical sensors in both the liquid and gas phases 17, 18.
The combination of optical fibre based sensing platforms with MIPs has already been attempted by several research groups. Previous reports have exploited surface plasmon resonance on metal coated optical fibres21, 22 or have used a fluorescence signal23, rather than LPGs. In all of these examples the MIP layer was produced in situ on the fibre, making large scale production of the optical sensors more challenging because of the need to control polymerization conditions to achieve high quality and reproducible MIP layers. In this paper, the MIP layer was deposited onto the optical fibre by simply immersing the optical fibre into a solution of soluble and easy-to-manipulate MIP nanoparticles. The nanoMIPs-modified optical fibre LPG was used to measure vancomycin in a concentration range from 10 nM to 700 μM, equivalent to 14 μg/L and 1 g/L, respectively. The lower concentration was chosen with the intention of assessing if this sensor was capable of measurements in dilutions of real samples, which would be helpful to reduce interferences when testing biological samples like plasma. The mid-range includes the recommended therapeutic concentration range, while the higher concentration was investigated to assess the concentrations at which the sensor response saturates. The selectivity of the sensor was tested against other commonly prescribed antibiotics such as gentamicin, amoxicillin and bleomycin24. In addition, to check the sensor performance in a complex biological matrix, the response to vancomycin was also measured in porcine plasma spiked with the antibiotic.
2. Experimental section
2.1 Materials
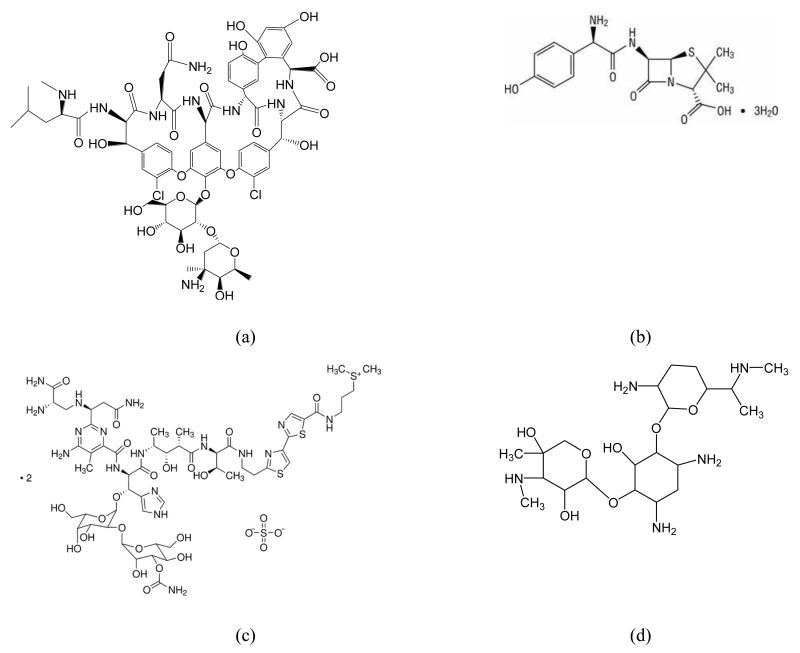
N -isopropylacrylamide (NIPAm), acrylic acid (AA), N, N’ methylene-bis-acrylamide (BIS), N-tert-butylacrylamide (TBAm), ammonium persulfate (APS), tetramethylethylenediamine (TEMED), 3-aminopropyltrimethyloxysilane (APTMS), glutaraldehyde (GA), ethanolamine, phosphate buffered saline (PBS), methanol, ethanol, toluene and acetone were purchased from Sigma-Aldrich, UK. The antibiotics vancomycin (VA), amoxicillin, bleomycin sulphate salt, gentamicin sulphate salt (Scheme 1) were also from Sigma-Aldrich. N-(3-aminopropyl)methacrylamide hydrochloride (APMA) was from Polysciences Europe GmbH, Germany. All the chemicals were of analytical grade, and used without further purification. Double-distilled ultrapure water (Millipore, UK) was used for the experiments. Phosphate buffered saline (PBS) was prepared as directed from PBS buffer tablets (Sigma- Aldrich, Gillingham, UK) and comprised phosphate buffer (0.01 M ), potassium chloride (0.0027 M ) and sodium chloride (0.137 M ), pH 7.4, at 25 ° C. Where PBS, pH 7.2, was used, the pH was adjusted by the addition of HCl.
2.2 Synthesis of MIP nanoparticles (nanoMIPs) imprinted with vancomycin
The protocol for the immobilization of vancomycin on solid supports (glass beads) has been described elsewhere8. An automated reactor, upgraded compared to that used previously8, was utilized for the synthesis of the nanoparticles. During the entire synthesis the template remains attached to the glass beads. Once the high affinity nanoparticles, formed around the template, are collected with hot washing, the template still remains attached to the solid surface and the nanoparticles collected are already ‘template free’. This is one of the major advantages of the solid-support approach in comparison with other methods used for the synthesis of nanoparticles. Regarding the details of the automated synthesis, the reactor, manufactured by HEL Ltd., (Borehamwood, UK), was designed to allow both photochemically- and chemically-initiated polymerizations. All processes were controlled with a computer, with minimal intervention from the operator. For the preparation of nanoMIPs specific for vancomycin, the polymerization mixture, which was adapted from that published by Hoshino and colleagues25 consisted of NIPAm (39 mg), BIS (2 mg), TBAm (33 mg dissolved in 2 mL of ethanol), APMA (1.15 mg) and AA (2.23 g). The mixture was dissolved in double distilled water (100 ml), sonicated for 10 minutes and degassed by bubbling with nitrogen for 30 minutes. For the automated synthesis of nanoMIPs, glass beads functionalized with vancomycin (60 g) were added to the reaction cylinder. Using a syringe pump, an aliquot of the polymerization mixture (60 mL) was added directly into the cylinder and then stirred briefly to homogenize the contents. Polymerization was initiated by the addition of a solution of APS (60 mg mL−1, 600 μL) and TEMED (18 μL) followed by stirring. The monomer mixture was left to polymerize at ambient temperature for 1.5 hours and a brief stirring (30 s at 600 RPM) was applied at the end of each 30 min period. At the end, the polymerization mixture was drained from the reaction vessel using a combination of suction and nitrogen purging. Subsequently, washings were performed at ambient temperature by adding water (2 aliquots of 50 mL) to the cylinder. The contents were stirred and the washings, containing unreacted monomers and other low affinity materials, removed and discarded. Next, a further 3 aliquots of water (50 mL each) were individually added to the cylinder and the temperature was raised to 60 °C. At this temperature pure fractions of nanoparticles were collected. The entire process took approximately 3.5 hours. The stages of the synthesis were pre-programed into the WinISO® software such that, once started, the synthesis could be left to run without the necessity for user supervision. The solution of nanoMIPs was concentrated to a final volume of 100 mL by ultrafiltration on a Millipore Amicon Ultra centrifugal filter unit (30 kDa MWCO) and its concentration (0.2 mg/ml) determined by weighing a freeze-dried aliquot. The size of the nanoparticles measured by dynamic light scattering (DLS) using a Zetasizer Nano (Nano-S) from Malvern Instruments Ltd (Malvern, UK) was around 280 nm. Prior to DLS measurements, performed at 25 °C, the solution of nanoMIPs was subjected to sonication for 5 minutes.
2.3 Sensor fabrication and functionalization
An LPG with a length of 30 mm and a period of 111 μm was fabricated in boron–germanium co-doped single mode optical fibre (Fibercore PS750) with a cut-off wavelength of 670 nm, in a point-by-point fashion, side-illuminating the optical fibre by the output from a frequency-quadrupled Nd:YAG laser, operating at 266 nm. The period of the LPG was chosen to ensure operation at the phase matching turning point, a condition known to offer the highest sensitivity26. The fibre was fixed tightly in a Teflon cell as described previously15-18. For transmission spectra (TS) measurements, one proximal end of the fibre was connected to a spectrometer (S200, Ocean Optics) and the distal end to a tungsten halogen lamp (HL-2000, Ocean Optics).
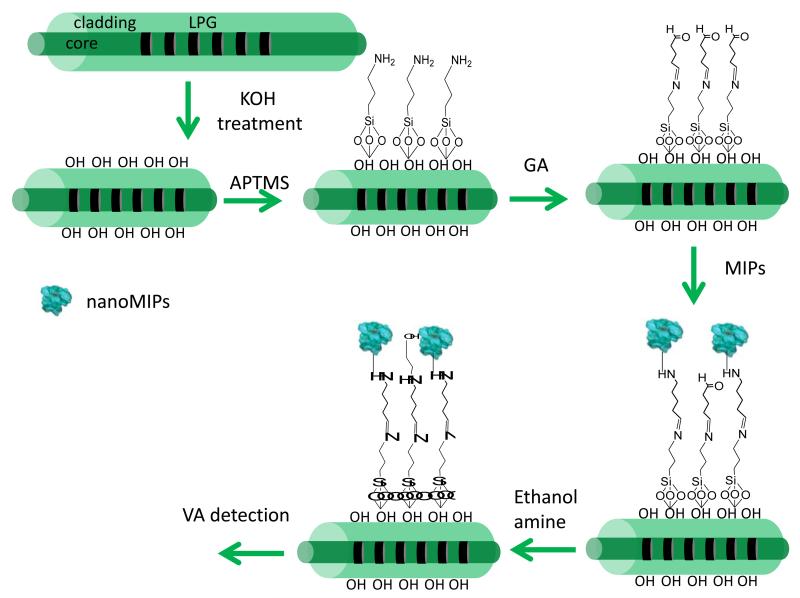
Prior to the deposition of the nanoMIPs, the region of the optical fibre containing the LPGs was rinsed with double-distilled water and immersed into 1 wt% ethanolic KOH (ethanol/water = 3:2, v/v) for 20 min, leading to formation of hydroxyl groups on the fibre surface. The fibre was then immersed into a 2% v/v solution of APTMS in dry toluene for different time intervals to find the optimal conditions, washed with acetone and subsequently incubated in a 1% v/v solution of GA in PBS (pH 7.2) for 1 hour. The LPG fibre was then rinsed with double-distilled water and immersed into a nanoMIPs solution (0.2 mg/ml) for 2 h. After rinsing with double distilled water, the fibre was immersed for 30 minutes into an ethanolamine water solution (0.1 M), to block the remaining free carbonyl groups of GA, and following a final wash with double-distilled water, the sensor was ready to be used. A schematic representation of the nanoMIPs immobilization protocol is reported on Scheme 3. The transmission spectrum (TS) of the LPG was measured after each step of the surface treatment.
Scheme 3.
Schematic illustration of the protocol used to immobilize nanoMIPs
2.4 Sensitivity and selectivity tests
After the functionalization, the sensor was exposed to variable concentrations of VA by immersion into 600 μL solution for 45 min, as after this time there was no change in measured signal. This was followed by thorough rinsing with distilled water and drying by purging with dry nitrogen to remove any physically adsorbed compounds. The transmission spectrum (TS) of the LPG was measured after each exposure to analyte and interfering substances. To avoid issues with sensitivity of the LPG to the bulk refractive index, all TS measurements were conducted in air. In order to take into account the potential difference in the light source intensity between different measurements the TS were normalised by taking an automatic baseline for each measured TS using Origin 7.5 software. After each exposure, a solution of NaOH (0.1 M) was used to regenerate the sensor by removing the VA adsorbed to the nanoMIPs. The sensor selectivity was tested by exposure to other common antibiotics (amoxicillin, gentamicin and bleomycin) with a concentration of 600-700 μM. The nanoMIPs-modified LPG was also immersed into porcine plasma spiked with 10 μM of vancomycin to check the sensor performance in a complex biological matrix.
It should be noted that the effect of the non-imprinted nanoparticles was thoroughly studied in the previous work with ELISA assay10 where the imprinting effect of the nanoMIPs was clearly demonstrated and was not included in this current study.
3 Results and discussion
3.1 NanoMIPs deposition
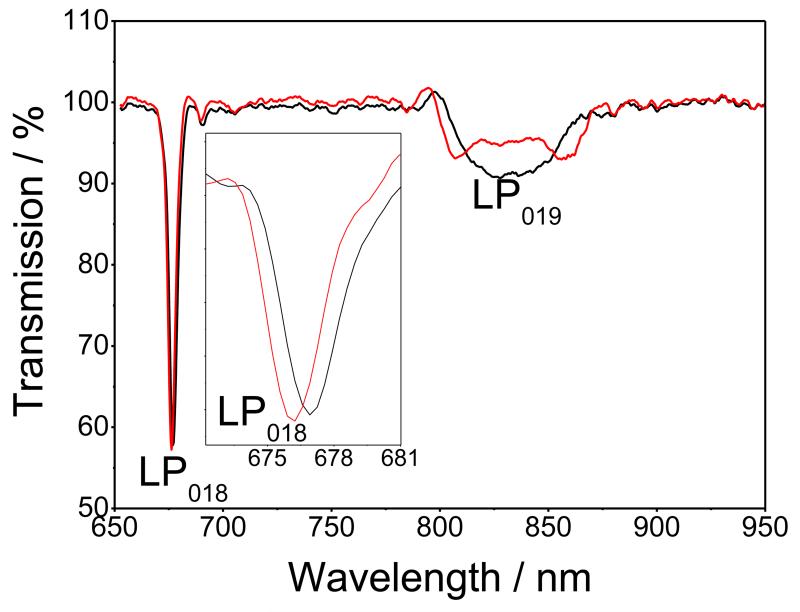
The first step to immobilize covalently nanoMIPs onto the optical fibre involved the deposition of a thin silane film onto the hydroxyl groups on the fibre surface formed by the KOH ethanolic solution. To do this the fibre was immersed into 2% v/v solution of APTMS in dry toluene. The silane also provides the surface with the primary amino groups necessary for the subsequent immobilization steps. Figure 1 shows the TS of the LPG with grating period of 111 μm, measured in air before and after 1 hour deposition of the APTMS. It should be noted that, in order to take into account the potential difference in the intensity of the light source between different measurements (Figure S1), the TS were normalised by taking an automatic baseline for each measured TS using Origin 7.5 software. The blue shift of the linearly polarised (LP) mode LP018 resonance, along with the appearance of the dual resonance for the LP019 mode resonance, is characteristic of the response of the LPG to the deposition of a thin film onto the cladding of the fibre.
Figure 1.
TS of the LPG measured in air: black line, before APTMS deposition, and red line, after APTMS deposition
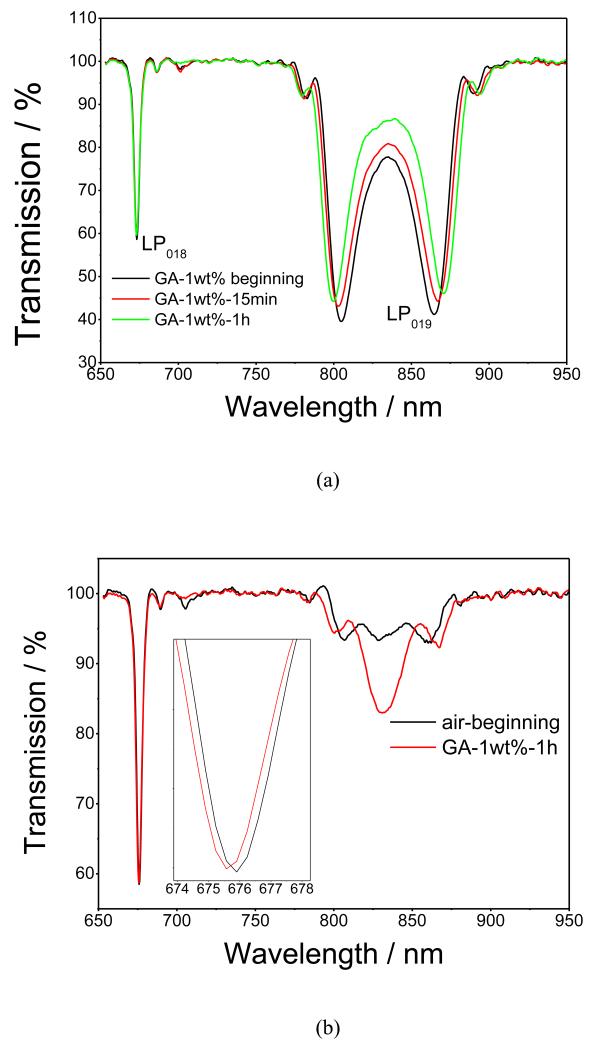
Following the deposition of the silane, the fibre was immersed into a PBS solution of GA, Figures 2a and 2b. Similarly to that observed following the deposition of the APTMS film, a further blue shift of the LP018 resonance, and further development of the LP019 dual resonance bands were observed, demonstrating a successful deposition of GA via attachment of the carbonyl groups of GA to the amino groups of the silane. When measured in solution, the LP019 resonance band exhibited a significant wavelength shift during the first 15 min from the start of the GA deposition, with the response saturating at around 30 min, as shown in Figure 2a. Figure 2b shows the TS measured in air before and after GA deposition, with the changes in the spectra indicating an increase of the optical thickness of the film.
Figure 2.
(a) Evolution of the TS of the APTMS-coated optical fibre LPG during the deposition of 1wt % GA, measured in solution at different interval times: black line, before GA deposition; red line, 15 min; and green line, 1 h after GA deposition; (b) TS measured in air: black line, before GA deposition, and red line, after GA deposition;
After GA deposition, the LPG was immersed into a water solution of nanoMIPs (0.2 mg/ml) for 2 hours, Figure S2. The amino groups contained in the NanoMIPs (due to the monomer APMA) create a bond with the carbonyl groups of GA. The minor change in the LP018 resonance band apparent after the immobilization of nanoMIPs can be explained by successful attachment of the nanoparticles to the GA surface. The final step of the MIP sensor fabrication was the blocking by ethanolamine (0.1 M) of any available carbonyl groups remaining on GA, Figure S3. A small change in the TS was observed, revealing the adsorption of the ethanolamine.
3.2 Vancomycin detection
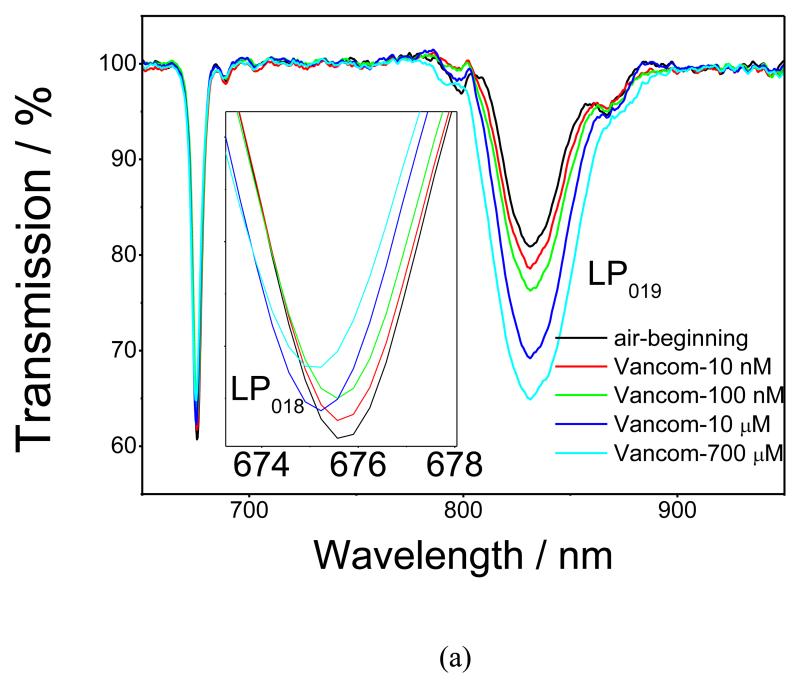
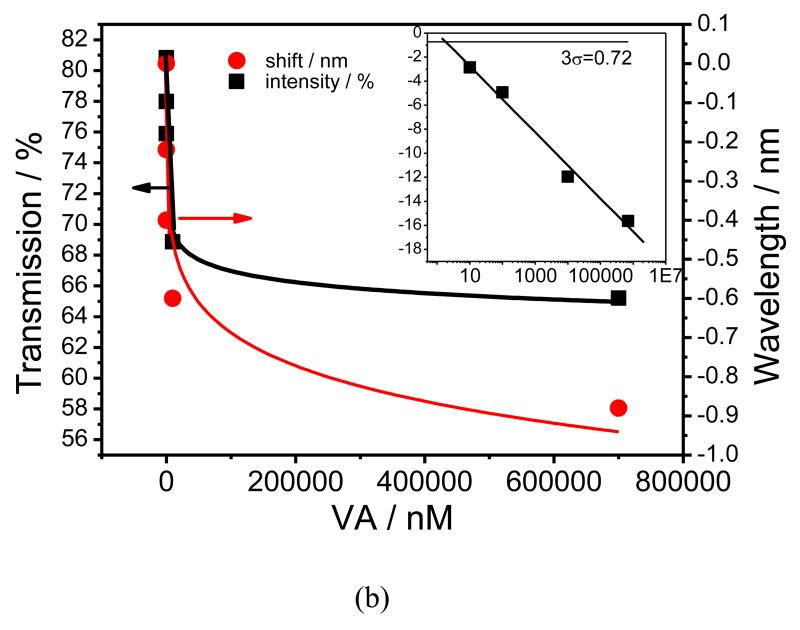
After the immobilization of the nanoMIPs and blocking with ethanolamine, the LPG was exposed to increasing concentrations of VA solution. Each concentration was incubated for 45 minutes (Figure 3a) as, after this time, the single was saturated, Figure S4. It should be noted, however, that these data cannot be directly used to assess amount that was bound, as the refractive index of the solution plays some role in the sensor response. The LPG was washed with 0.1 M NaOH after each exposure (Figure S5). As the concentration of VA increased, the change in the TS becomes more pronounced, indicating an increase in the amount of VA bound to the nanoMIPs. This behaviour of the TS of the coated LPG fibre is typical of that observed when the RI of the sensitive layer increases15-18. The resonance band at ca. 670 nm, which is coupled to the linearly polarized 18th cladding mode (LP018), exhibits blue shifts of 0.22, 0.40, 0.60 and 0.88 nm for the 0.01, 0.1, 10 and 700 μM VA concentrations, respectively. The transmission intensity of the resonance band at ca. 833 nm, corresponding to coupling to the LP019 modes, decreases for 2.85, 4.93, 11.96 and 15.63 % for 0.01, 0.1, 10 and 700 μM VA concentrations, respectively, Figure 3a. The sensor response starts to saturate at a concentration of VA of 700 μM, indicating the specific nature of VA binding, Figure 3b. The main focus of the sensor characterisation was on concentrations lower than the physiological range (10 nM), demonstrating that this approach could be used to develop a sensor with sensitivity that would need dilutions of real samples before testing. This approach would have the effect of reducing interferences when testing biological samples like plasma.
Figure 3.
(a), TS of the nano-MIP coated LPG measured in air after exposure to VA concentrations ranging from 10 nM to 700 μM; (b), Dependence of the transmission at the centre of the resonance band corresponding to coupling to the LP019 mode (square) and wavelength shift of the resonance band corresponding to coupling to the LP018 mode (circle) on the concentration of VA. The red and black lines are fits using the Langmuir adsorption isotherm. The inset shows the logarithmic dependence of transmission at the centre of the resonance band corresponding to coupling to the LP019 mode on VA concentration.
When the nanoMIP-modified LPG was regenerated with NaOH (0.1 M) the signal returned to its original value, indicating total removal of VA from the nanoMIPs, Figure S5. The binding of VA to the nanoMIPs was fitted with a Langmuir model using the standard package available in Origin software (Figure 3b and S6). The binding constant of VA to the nanoMIPs film, calculated using a Scatchard-Rosenthal plot in accordance with the Eq 2 (Figure S5), was 4.3±0.01×10−8 M27.
| (2) |
where Bmax is themaximum amount of VA bound (sensor response at highest concentration), B is the amount of VA bound at a given concentration (sensor response at a given concentration), Kd is the binding constant and c is the concentration of VA.
The standard deviation, σ, obtained from 5 exposures to blank sample (Figure S6) and 10 μM of VA, was ±0.24 % and ±0.12 nm for the transmission and wavelength measurements, respectively. For transmission measurements, the limit of detection of the sensor system was found to be 1.8 nM, determined by calculating the 3σ value27.
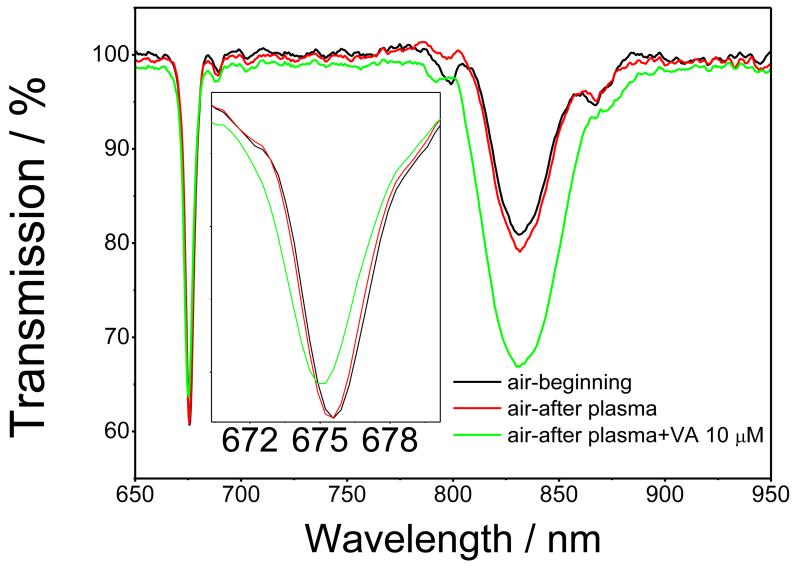
Figure 4 shows the TS measured in air before and after exposure (for 45 minutes) to porcine plasma with and without VA (10 μM). A small sensor response was observed for plasma without VA. The response increased when 10 μM of VA was present in the plasma, Figure 4. These results suggest that the sensor can reliably perform VA detection in a complex medium such as plasma. It should be noted, however, that response to 10 μM of VA was larger when measured in plasma as compared to VA in PBS. The most plausible explanation for this is the effect of the complex medium on the sensor response; this phenomenon could be solved either by further optimisation of the blocking of the particles or by performing an antibiotic calibration curve directly in plasma which should negate the effect of the complex medium. This will be studied in more detail in future work.
Figure 4.
TS of the nano-MIP coated LPG, measured in air before (black line) and after exposure to, porcine plasma (red line) and porcine plasma spiked with 10 μM of VA (green line)
3.3 Selectivity test
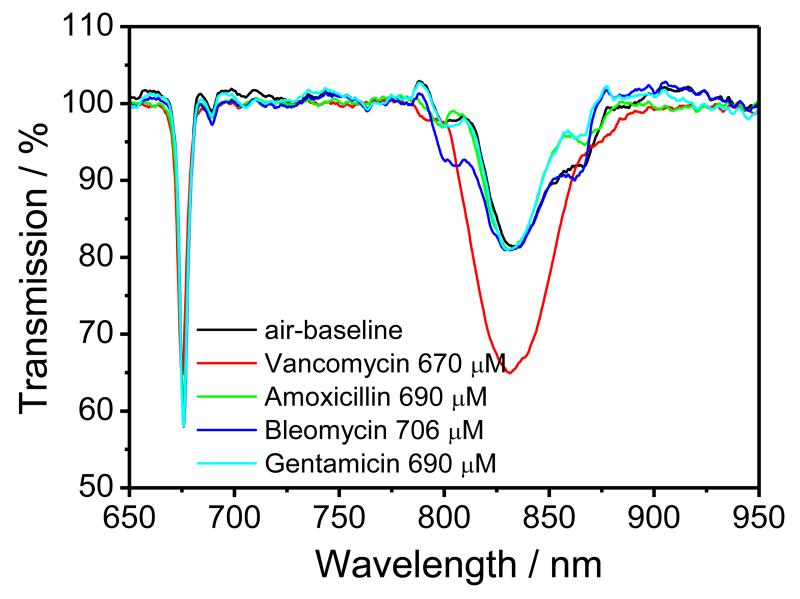
When the nanoMIPs-modified LPG was exposed to solutions of amoxicillin (610 μM), bleomycin (700 μM) and gentamicin (660 μM), each prepared in PBS, no measurable changes in the central wavelength of the resonance band corresponding to coupling to the LP018 mode, nor of the transmission at the centre of the resonance band corresponding to coupling to the LP019 mode were observed, revealing the high selectivity of the LPG sensor towards other commonly prescribed antibiotics, (Figure 5).
Figure 5.
TS of the nano-MIP-coated LPG, measured in air after exposure to, VA (670 μM) (red line), amoxicillin (690 μM) (green line); bleomycin (706 μM) (blue line) and gentamicin (690 μM) (magenta line).
Conclusions
In conclusion, molecularly imprinted polymer nanoparticles specific for VA were successfully deposited for the first time onto an optical fibre long period grating. The sensor demonstrated high sensitivity (10 nM with 4.3±0.01×108 M−1 binding constant) to VA and selectivity over other antibiotics such as amoxicillin, bleomycin and gentamicin. Successful detection of VA in a complex biological matrix, porcine plasma was also demonstrated. The LPG sensor based on molecular imprinted nanoparticles presented here is a promising tool for detection of antibiotics in biological fluids. In addition, the generic nature of both the LPG sensor and the nanoMIP prepared by solid-phase synthesis suggests that by immobilizing different nanoMIPs (produced against a different analyte), low cost and easy to use optical fibre sensors can be produced for applications in a variety of fields, from medical and environmental to food and industry.
Supplementary Material
Scheme 2.
Schematic illustration of the chemicals used for detection and selectivity tests: (a) vancomycin (VA); (b) amoxicillin; (c) bleomycin and (d) gentamicin.
Acknowledgment
The authors thank The Wellcome Trust (UK) for the financial support through the granting of a Translational Award and the EPRSC Platform Grant (EP/H02252X/1).
References
- 1.Goncalves HMR, Duarte AJ, Davis F, Higson SPJ. Analytica Chimica Acta. 2012;735:90. doi: 10.1016/j.aca.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Consales M, Campopiano S, Cutolo A, Penza M, Aversa P, Cassano G, Giordano M, Cusano A. Measurement Science and Technology. 2006;17:1220. [Google Scholar]
- 3.Lee S-W, Korposh S, Selyanchyn R, Kunitake T. In: Handbook of Molecular Imprinting: Advanced Sensor Applications. Lee S-W, Kunitake T, editors. Pan Stanford Publishing Pte Ltd; 2012. pp. 1–64. ch. 1. [Google Scholar]
- 4.Whitcombe MJ, Chianella I, Larcombe L, Piletsky SA, Noble J, Porter R, Horgan A. Chemical Society Reviews. 2011;40:1547. doi: 10.1039/c0cs00049c. [DOI] [PubMed] [Google Scholar]
- 5.Malitesta C, Mazzotta E, Picca RA, Poma A, Chianella I, Piletsky SA. Analytical and Bioanalytical Chemistry. 2011;402:1827. doi: 10.1007/s00216-011-5405-5. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Diaz G, Antuna- Jimenez D, Blanco-Lopez MC, Lobo-Castanon MJ, Miranda-Ordieres AJ, Tunon-Blanco P. Trends in Analytical Chemistry. 2012;33:68. [Google Scholar]
- 7.Uludag Y, Piletsky SA, Turner APF, Cooper MA. FEBS Journal. 2007;274:5471. doi: 10.1111/j.1742-4658.2007.06079.x. [DOI] [PubMed] [Google Scholar]
- 8.Poma A, Guerreiro A, Whitcombe MJ, Piletska EV, Turner APF, Piletsky SA. Advanced Functional Materials. 2013;23:2821. doi: 10.1002/adfm.201202397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moczko E, Poma A, Guerreiro A, Perez de Vargas Sansalvador I, Caygill S, Canfarotta F, Whitcombe MJ, Piletsky S. Nanoscale. 2013;5:3733. doi: 10.1039/c3nr00354j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chianella I, Guerreiro A, Moczko E, Caygill JS, Piletska EV, Perez De Vargas Sansalvador IM, Whitcombe MJ, Piletsky SA. Anal. Chem. 2013;85:8462. doi: 10.1021/ac402102j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP. Am J Health-Syst Pharm. 2009;66:82. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 12.Truong J, Levkovich BJ, Padiglione AA. Internal Medicine Journal. 2012;42:23. doi: 10.1111/j.1445-5994.2011.02459.x. [DOI] [PubMed] [Google Scholar]
- 13.de Jesus Valle MJ, Gonzalez Lopez F, Sanchez Navarro A. Journal of Pharmaceutical and Biomedical Analysis. 2008;48:835. doi: 10.1016/j.jpba.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Rodvold KA, Erdman SM, Pryka RD. In: Therapeutic drug monitoring. Schumacher GE, editor. Appleton and Lange; Norwalk, CT: 1995. pp. 587–632. [Google Scholar]
- 15.Simons SA, Molinelli AR, Sobhani K, Rainey PM, Hoofnaglea AN. Clinical Chemistry. 2009;55:578. doi: 10.1373/clinchem.2008.112946. [DOI] [PubMed] [Google Scholar]
- 16.Korposh S, Lee S-W, James SW, Tatam RP. Meas. Sci. Technol. 2011;22:075208, 10. [Google Scholar]
- 17.Korposh S, James SW, Lee SW, Topliss S, Cheung SC, Batty WJ, Tatam RP. Optics Express. 2010;18:13227. doi: 10.1364/OE.18.013227. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Korposh S, Wong R, James S, Tatam R, Lee SW. Chem. Lett. 2012;41:1297. [Google Scholar]
- 19.Korposh S, Wang T, James S, Tatam R, Lee SW. Sens. Act. B. 2012;173:300. [Google Scholar]
- 20.Falciai R, Mignani AG, Vannini A. Sens. Actuators B. 2001;74:74. [Google Scholar]
- 21.Verma R, Gupta BD. Sens. Actuators B. 2013;177:279. [Google Scholar]
- 22.Cennamo N, D’Agostino G, Galatus R, Bibbo’ L, Pesavento M, Zeni L. Sens. Actuators B. 2013 DOI: 10.1016/j.snb.2013.07005. [Google Scholar]
- 23.Nguyen TH, Hardwick SA, Tong S, Grattan KTU. IEEE Sensors Journal. 2012;12:255. [Google Scholar]
- 24.Fujiwara K, Yoshizaki Y, Shin M, Miyazaki T, Saita T, Nagata S. Antimicrobial Agents Chemotherapy. 2012;56:5883. doi: 10.1128/AAC.01267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshino Y, Urakami T, Kodama T, Koide H, Oku N, Okahata Y, Shea KJ. Small. 2009;5:1562. doi: 10.1002/smll.200900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung SC, Topliss SM, James SW, Tatam RP. Journal of the Optical Society of America B. 2008;25:897. [Google Scholar]
- 27.Bossi A, Piletsky SA, Piletska EV, Righetti PG, Turner APF. Anal. Chem. 2001;73:5281. doi: 10.1021/ac0006526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.