Abstract
Patient: Female, 51
Final Diagnosis: Transient ischemic attack
Symptoms: Unilateral left-sided weakness and slurred speech
Medication: Lisinopril 20 mg daily
Clinical Procedure: Transesophageal echocardiogram
Specialty: Cardiology • Neurology
Objective:
Rare disease
Background:
In 1856, a Bohemian physician, Vilém Dušan Lambl, first described the presence of filiform lesions in aortic valve leaflets. Lambl’s excrescences are tiny filiform strands that arise on the line of valve closure, and result from valvular wear and tear. It is a rare cause of cardioembolic stroke that can be detected by transesophageal echocardiogram.
Case Report:
We encountered a 51-year-old, African-American woman with a history of recurrent strokes that we suspect may be the result of Lambl’s excrescence. The patient was treated with dual antiplatelet therapy and was recommended to have surveillance transesophageal echocardiograms at 6 months and 1 year from the time of discharge.
Conclusions:
As there are no definitive guidelines for the management of patients with Lambl’s excrescences, we present a review of the current medical literature and a specific case report in an attempt to provide a better strategy for managing this condition. In our case report, we focus on the management and treatment for Lambl’s excrescence because no clear evidence has been published in the literature. Our review indicates that Lambl’s excrescence, despite its relative scarcity, should be considered in the differential diagnosis of a patient with cryptogenic stroke.
MeSH Keywords: Heart Valves; Ischemic Attack, Transient; Stroke
Background
In 1856, Vilém Dušan Lambl, a Bohemian physician first described the presence of filiform lesions on aortic valve leaflets [1]. Lambl’s excrescences (LEs) are found on the line of closure of heart valves, and can result in mechanical endothelial damage. This condition is most often diagnosed incidentally by transesophageal echocardiogram (TEE) [2]. They carry a potential risk of embolizing to distant organs causing stroke [3–6] or myocardial infarction [7,8]. Histologically, they are composed of fibroelastic and hyalinized stroma covered by a layer of endothelial cell lining [3]. They are similar histopathologically to cardiac papillary fibroelastomas (CPFs). They differ in that CPFs are larger and are found away from the line of valve closure, whereas LEs are found at the line of valve closure [9].
Although the most common causes of cardioembolic stroke include atrial fibrillation, valvular heart disease, patent foramen ovale, and left ventricular dysfunction [10], LE is not commonly considered in the list of potential causes of stroke. LEs are hypothesized to form as a result of valvular wear and tear. Once formed, they can be a source of microthrombi [10]. Since the first description of LE involving the aortic valve in 1856, only several cases have been reported in the medical literature. An evidence-based approach to the management and treatment of LE has yet to be elucidated. We report a case of a 51-year-old woman with a history of 2 prior cerebral vascular accidents, who presented to the emergency department with transient ischemic attack (TIA) symptoms.
Case Report
A 51-year-old, right-handed, African-American woman presented to the emergency room with transient symptoms of worsening baseline left-sided weakness and slurred speech. Her past medical history was significant for well-controlled hyper-tension for 11 years, 2 ischemic strokes, and the presence of LE on the aortic valve on TEE. She was also a former tobacco abuser prior to February 2013. A review of her medical record revealed 2 prior hospitalizations. During her first hospitalization in February 2013, the patient was admitted for an ischemic stroke. Brain magnetic resonance imaging (MRI) showed multiple areas of acute ischemia, including the right corona radiata and centrum semiovale, with multiple foci of acute ischemia in the gray-white matter interface on the right hemisphere (Figures 1, 2). Her medical workup included an electrocardiogram (EKG), carotid Doppler, and transthoracic echo-cardiogram (TTE) with bubble study, all of which were negative. Aortography and 4-vessel cerebral angiography done did not show atherosclerotic plaque or occlusive disease. Her lipid profile showed total cholesterol 142 mg/dL, low-density lipoprotein (LDL) 83 mg/dL, high-density lipoprotein (HDL) 36 mg/dL, and triglyceride 114 mg/dL. She was diagnosed with a cryptogenic stroke and was discharged home with aspirin 81 mg daily and pravastatin 40 mg daily. Subsequently, the patient underwent outpatient cardiac monitoring with an implantable loop recorder, which did not reveal cardiac arrhythmia. During her second hospitalization 3 months later (May 2013), the patient presented with a transient episode of left-sided weakness and slurred speech. An MRI brain with diffusion revealed a resolving old ischemic infarct with new areas of punctate acute ischemia in the posterior supratentorial brain at the level of the centrum semiovale (Figure 3). TEE at that time revealed a 2-mm wavy strand on the aortic valve protruding into the ascending aorta, suggestive of an LE. There was no evidence of valvular vegetation (Figures 4, 5). The left atrium was normal in size, and no thrombus was detected in the left atrium or left atrial appendage. She was discharged home and instructed to take aspirin 81 mg daily and clopidogrel 75 mg daily.
Figure 1.
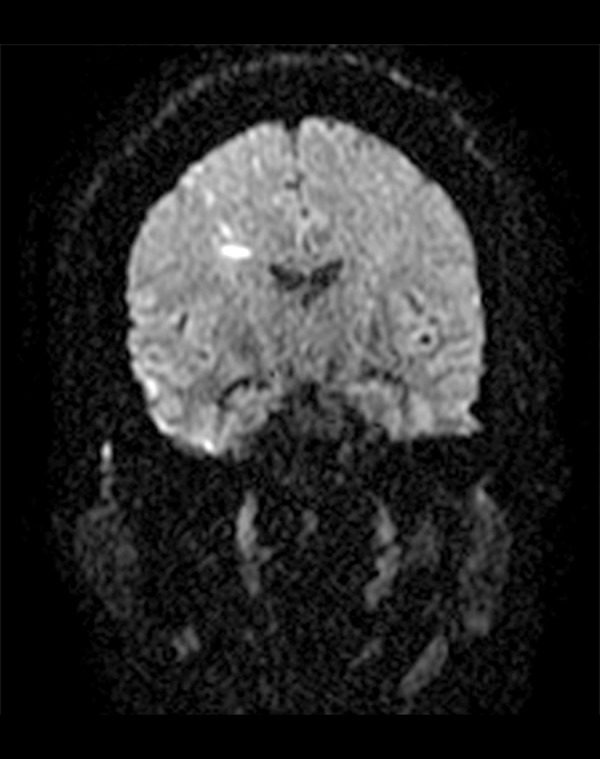
MRI brain diffusion scan coronal view. Multiple areas of acute ischemia in the right corona radiata centrum semiovale with multiple foci of ischemia in the gray-white matter interface on the right.
Figure 2.
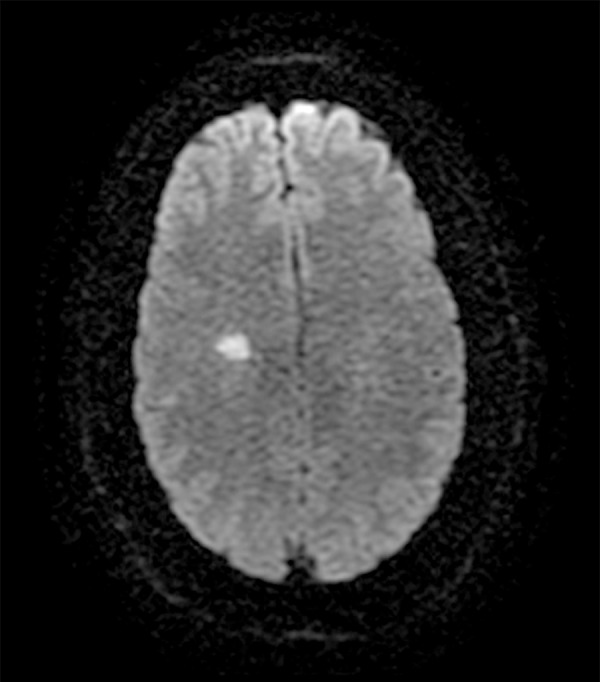
MRI brain diffusion scan axial view. Area of acute ischemia in the right corona radiata centrum semiovale.
Figure 3.
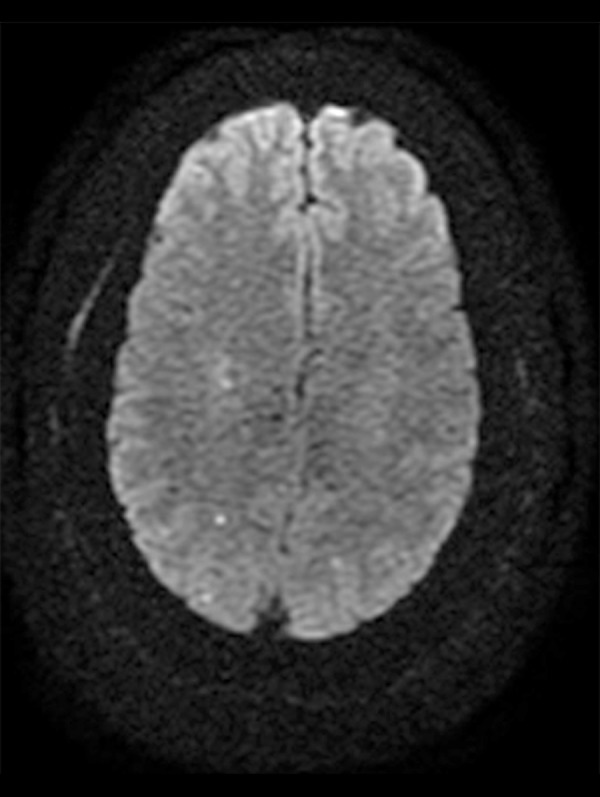
MRI brain axial view with diffusion revealed a resolving old ischemic infarct with new areas of punctuate acute ischemia in the posterior supratentorial brain at the level of centrum semiovale.
Figure 4.
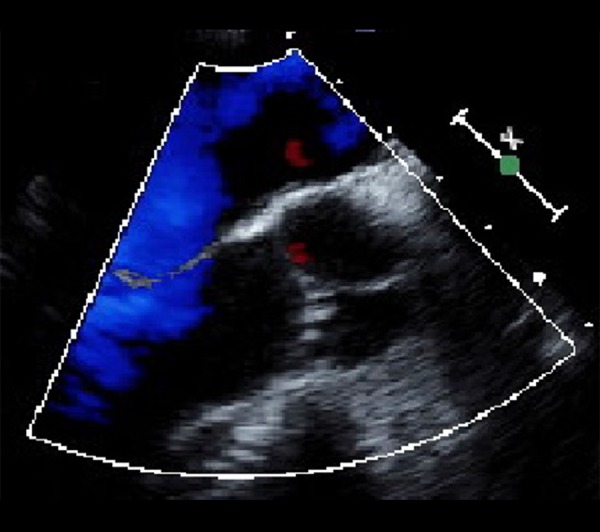
Transesophageal echocardiogram showing a small strand on the aortic valve protruding into the ascending aorta.
Figure 5.

Transesophageal echocardiogram showing the small Lambl’s excrescence (arrow), LV (Left Ventricle), AV (Aortic Valve), A (Aorta).
The patient returned to the emergency department several months later with similar symptoms of transient left-sided weakness. It was noted that the patient was not compliant with the dual antiplatelet therapy in the prior month. Her symptoms resolved upon initial evaluation. She scored 2 points on the NIH Stroke Scale. On physical examination her vital signs were stable and the neurological examination was consistent with diminished strength intensity of 4/5 in both proximal and distal left upper and lower extremities. This was consistent with her baseline motor function. All her laboratory values were normal, including a lipid panel, hypercoagulability workup, and troponins. Bilateral carotid Doppler ultrasonography showed a stable carotid plaque in the common carotids with no evidence of a hemodynamically significant stenosis on either side. MRI of the brain showed improvement of her prior ischemic strokes, but no evidence of a new ischemic event. TTE did not show any potential cardioembolic source. Since the MRI failed to reveal any focal finding, the multidisciplinary team decided to resume dual antiplatelet therapy with aspirin 81 mg daily and clopidogrel 75 mg daily.
At her 3-month and 6-month follow-up visits, the patient had no further neurological events to suggest ongoing thromboembolic risk. Her symptoms of left-sided weakness remained essentially unchanged. The patient reported compliance with her medications during this time period. She also had a repeat TEE at 6 months, which showed a stable size LE. We advised the patient to continue with lifelong dual antiplatelet therapy and to repeat TEE in 12 months to evaluate the stability of the LE.
Discussion
Only several cases of LE have been reported in the medical literature. We performed a review of published literature using PubMed to identify previously published cases of LE from 1981 up to 2014 (Table 1) [2–6,8,11–24]. Aortic and mitral valves were the two most common locations of LE. However, ischemic events were more commonly observed in association with LEs on aortic valves [3,5,6,8,15–17,20,21,23,24] as compared to mitral valves [4,13]. Although there has been some case reports on giant LEs (≥2 cm in diameter) associated with ischemic stroke [6,15], no clear evidence exists in the literature showing the correlation between the strand size and potential risk of thromboembolic event.
Table 1.
Previously reported cases of Lambl’s excrescence.
| Reference | Patient demographics | Location | Presentation | Management | Follow-up outcome |
|---|---|---|---|---|---|
| Cha SD [11] (1981) | 54-year-old female | Papillary muscle and aortic valve | Incidental finding | Surgical excision | Not reported |
| Fitzgerald D [12] (1982) | 70-year-old male | unknown | Embolus to popliteal artery | Surveillance echocardiogram | Uneventful for 18-month periodic echocardiogram follow-up |
| Nighoghossian N [13] (1995) | 31-year-old male | Mitral valves | Recurrent ischemic stroke | Acenocoumarol (1st time) Surgical excision (2nd time) |
Not reported |
| Quinson P [8] (1996) | 64-year-old female | Aortic valve | Angina | Surgical excision Aspirin 250 mg | Symptom-free at 3 months follow-up |
| Voros S [2] (1999) | 72-year-old male | Pulmonary valve | Ischemic stroke | Conservative management | Not reported |
| Berent R [14] (1998) | 44-year-old female | Aortic valve | Weakness and Fatigue | Surgical excision | Not reported |
| Aggarwal A [15] (2003) | 66-year-old female | Aortic valve | Ischemic strokes | Anticoagulation (1st time) Surgical excision (2nd time) |
Not reported |
| Wolf RC [16] (2006) | 47-year-old female | Aortic valve | Ischemic stroke | Aspirin 100 mg | No neurological event at 6-month follow-up |
| Siles RJR [17] (2006) | 42-year-old male | Aortic valve | Transient ischemic attack | Aspirin 200 mg | No neurological event at 1 year follow-up |
| Aziz F [3] (2007) | 61-year-old female | Aortic valve | Ischemic stroke | Surgical excision | No neurological event at 1 year follow-up |
| Jaffe W [18] (2007) | 80-year-old female | Aortic valve | Incidental finding | Not Report | Not reported |
| Nakahira J [19] (2008) | 69-year-old female | Aortic valve | Incidental finding | Surgical excision | Not reported |
| Kalavakunta JK [5] (2010) | 59-year-old male | Aortic valve | Ischemic stroke | Warfarin therapy | Not reported |
| Mito M [20] (2012) | 50-year-old female | Aortic valve | Myocardial Infarction | Expired | – |
| Liu RZ [21] (2012) | 53-year-old male | Aortic valve | Headache and ischemic stroke | Aspirin 100 mg | Symptom-free at 9-month follow-up |
| 30-year-old male | Aortic valve | Headache and ischemic stroke | Surgical excision | Symptom-free at 7-month follow-up | |
| Morgan JA [22] (2012) | Female | Aortic valve | Incidental finding | Surgical excision | Not Reported |
| Wu TY [6] (2013) | 66-year-old female | Aortic valve | Ischemic stroke | Lifelong warfarin therapy | No neurological event at 6-month follow-up |
| Al-Ansari S [4] (2013) | 33-year-old male | Mitral valve and chordae tendineae | Ischemic stroke | Surgical excision | Not reported |
| Yacoub HA [23] (2014) | 59-year-old male | Aortic valve | Ischemic stroke | Aspirin and Lipitor | Not reported |
| Davogustto G [24] (2015) | 68-year-old female | Aortic valve | Migraine headache and ischemic stroke | Aspirin | No neurological event at 2-year follow-up |
| Current case | 51-year-old female | Aortic valve | Ischemic stroke (1st & 2nd time) Transient ischemic attack (3rd time) |
Aspirin 81 mg and Clopidogrel 75 mg | No neurological event at 3-month and 6-month follow-up |
Whether LEs are involved in generating ischemic events remains controversial. In the only prospective study in our review, Roldan et al. [25] performed TEE assessment on healthy volunteers (Group 1: n=88), patients without suspected cardioembolic disease (Group 2: n=88) and patients undergoing TEE for suspected cardioembolic disease (Group 3: n=49). They found a similar prevalence of valve excrescences across all groups, suggesting that excrescences were not associated with cardioembolic phenomenon. This was further supported by their observation that the rate of cardioembolic events over approximately >4 years was similar in those with and without valve excrescences. Additionally, they noted that aspirin and warfarin therapy did not seem to affect the prevalence or natural history of valve excrescences. However, due to the relatively small sample size, low event rates, and lack of histological confirmation of the type of excrescence, the authors were unable to definitively exclude LE as a possible source of cardioembolic events. Their findings are in contrast to those of Freedberg et al., who noted, in a retrospective analysis, a higher prevalence of excrescences in patients with embolic phenomenon than those without (10.6% vs. 2.3%) [26].
Given the lack of consensus in literature, it is difficult to exclude LEs as a possible source of embolic phenomenon in patients with cryptogenic stroke. In our case, alternative causes were ruled out (negative EKG, hypercoagulability panel, carotid US, and arrhythmias), leading us to suggest that LEs may have been responsible for our patient’s presentation. This is in line with previous case reports documenting a similar association [3,4–6,13,15–17,21,23,24]. However, for our patient, the first episode of stroke was diagnosed as cryptogenic stroke and no TEE workup was done at that time. Therefore, we could not conclude that her stroke was associated with an undiagnosed LE. During her second episode of stroke, an LE was found on TEE and she was started on dual antiplatelet therapy. During the last hospitalization, our patient was diagnosed with TIA as there was no evidence of an infarct on brain MRI. Although our patient’s CHA2DS2-VASc score was 4, there was no evidence of atrial fibrillation revealed from her loop recorder. Therefore, we decided to instruct the patient to continue with dual antiplatelet therapy (aspirin 81 mg and clopidogrel 75 mg daily) since she had failed aspirin alone in the past. In the event our patient was to develop atrial fibrillation, switching from dual antiplatelet to anticoagulation therapy and excision of LE would be considered. We recommended our patient to have a surveillance TEE at 6 months and 1 year from the time of hospital discharge to visualize the stability of LE.
Conclusions
Further research is needed to evaluate the clinical importance of LE, including the pathophysiology, its association with ischemic events, and how to manage and treat it. It is our opinion that physicians should still consider LE in the differential diagnosis for a patient with cryptogenic stroke until conclusive evidence suggests otherwise. We recommend performing a TEE in this population, and if LE is present, we suggest that treatment with dual antiplatelet therapy should be considered. If there is a recurrent ischemic event while on this therapy, a trial of anticoagulation therapy should be considered before proceeding with surgical resection of the LE. Indication for the surgical resection of LE is still controversial, simply based on a few case reports [3,4,8,11,13–15,19,21,22] and poor follow-up outcomes. In conclusion, our case is in line with previous case reports; however, this underscores the need for well-designed studies to determine appropriate strategies for treating valve excrescences in these patients.
Acknowledgments
We thank Lauren Cohen-Sahalon for her contribution in manuscript preparation (review and editing).
References:
- 1.Lambl V. Papillare excrescenzen an der semilunar-klappe der aorta. Wien Med Wochenschr. 1856;6:1. [in German] [Google Scholar]
- 2.Voros S, Nanda NC, Thakur AC, et al. Lambl’s excrescences (valvular strands) Echocardiography. 1999;16:399–414. doi: 10.1111/j.1540-8175.1999.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 3.Aziz F, Baciewicz FA. Lambl’s excrescences: review and recommendations. Tex Heart Inst J. 2007;34:366–68. [PMC free article] [PubMed] [Google Scholar]
- 4.Al-ansari S, Hindori V, Riezebos RK, Yilmaz A. Multiple Lambl’s excrescences with subvalvular extension, a rare cause of cryptogenic stroke: treated by port-access cardiac surgery. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-201161. pii: bcr2013201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalavakunta JK, Peddi P, Bantu V, et al. Lambl’s excrescences: a rare cause of stroke. J Heart Valve Dis. 2010;19:669–70. [PubMed] [Google Scholar]
- 6.Wu TY, Gerber IL, Roxburgh RH. Thrombo-embolic cerebral infarction secondary to giant Lambl’s excrescence. J Clin Neurosci. 2013;20:1632–34. doi: 10.1016/j.jocn.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Dangas G, Dailey-sterling FG, Sharma SK, et al. Non-Q-wave infarction and ostial left coronary obstruction due to giant Lambl’s excrescences of the aortic valve. Circulation. 1999;99:1919–21. doi: 10.1161/01.cir.99.14.1919. [DOI] [PubMed] [Google Scholar]
- 8.Quinson P, De gevigney G, Boucher F, et al. [Fibrous aortic valve tumor (Lambl’s excrescence) trapped in the right coronary artery. Apropos of a case] Arch Mal Coeur Vaiss. 1996;89:1419–23. [in French] [PubMed] [Google Scholar]
- 9.Boone SA, Campagna M, Walley VM. Lambl’s excrescences and papillary fibroelastomas: are they different? Can J Cardiol. 1992;8:372–76. [PubMed] [Google Scholar]
- 10.Leary MC, Caplan LR. Cardioembolic stroke: An update on etiology, diagnosis and management. Ann Indian Acad Neurol. 2008;11(1):52–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Cha SD, Incarvito J, Fernandez J, et al. Giant Lambl’s excrescences of papillary muscle and aortic valve: echocardiographic, angiographic, and pathologic findings. Clin Cardiol. 1981;4:51–54. doi: 10.1002/clc.4960040112. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald D, Gaffney P, Dervan P, et al. Giant Lambl’s excrescence presenting as a peripheral embolus. Chest. 1982;81:516–17. doi: 10.1378/chest.81.4.516. [DOI] [PubMed] [Google Scholar]
- 13.Nighoghossian N, Trouillas P, Perinetti M, et al. [Lambl’s excrescence: an uncommon cause of cerebral embolism] Rev Neurol (Paris) 1995;151:583–85. [in French] [PubMed] [Google Scholar]
- 14.Berent R, Hartl P, Rossoll M, Punzengruber C. [Lambl’s excrescence as tumorous heart valve mass] Dtsch Med Wochenschr. 1998;123:423–26. doi: 10.1055/s-2007-1023981. [in German] [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal A, Leavitt BJ. Images in clinical medicine. Giant Lambl’s excrescences. N Engl J Med. 2003;349:e24. doi: 10.1056/ENEJMicm010900. [DOI] [PubMed] [Google Scholar]
- 16.Wolf RC, Spiess J, Huber R. [Lambl’s excrescence and cerebral ischemic insult] Nervenarzt. 2006;77:1492–94. doi: 10.1007/s00115-006-2198-4. [in German] [DOI] [PubMed] [Google Scholar]
- 17.Siles rubio JR, Ruiz de castroviejo del campo J, Tirado miranda R, et al. [Transient ischemic attack due to Lambl’s excrescence. Report of a case and review of the literature] An Med Interna. 2006;23:181–83. doi: 10.4321/s0212-71992006000400009. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 18.Jaffe W, Figueredo VM. An example of Lambl’s excrescences by transesophageal echocardiogram: a commonly misinterpreted lesion. Echocardiography. 2007;24:1086–89. doi: 10.1111/j.1540-8175.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakahira J, Sawai T, Katsumata T, et al. Lambl’s excrescence on aortic valve detected by transesophageal echocardiography. Anesth Analg. 2008;106:1639–40. doi: 10.1213/01.ane.0000313440.93243.6b. [DOI] [PubMed] [Google Scholar]
- 20.Mito M, Kiyuna M, Toda T, et al. [A rare case report of incarceration of Lambl’s excrescence of aortic valve resulting in myocardial infarction] Rinsho Byori. 2012;60:758–61. [in Japanese] [PubMed] [Google Scholar]
- 21.Liu RZ, Yu SY, Li Y. Migraine-like headache and ischemic strokes in two patients with Lambl’s excrescences. Chin Med J (Engl) 2012;125:3346–48. [PubMed] [Google Scholar]
- 22.Morgan JA, Paone G. Resection of Lambl’s excrescence on the aortic valve in a patient with rheumatic mitral valve disease and a left atrial thrombus. Heart Surg Forum. 2012;15:E215–17. doi: 10.1532/HSF98.20121023. [DOI] [PubMed] [Google Scholar]
- 23.Yacoub HA, Walsh AL, Pineda CC. Cardioembolic stroke secondary to Lambl’s excrescence on the aortic valve: a case report. J Vasc Interv Neurol. 2014;7:23–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Davogustto G, Fernando RR, Loghin C. Lambl’s excrescence, migrainous headaches, and “tiger stripes”: puzzling findings in one patient. Tex Heart Inst J. 2015;42:70–72. doi: 10.14503/THIJ-13-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roldan CA, Shively BK, Crawford MH. Valve excrescences: prevalence, evolution and risk for cardioembolism. J Am Coll Cardiol. 1997;30:1308–14. doi: 10.1016/s0735-1097(97)00315-x. [DOI] [PubMed] [Google Scholar]
- 26.Freedberg RS, Goodkin GM, Perez JL, et al. Valve strands are strongly associated with systemic embolization: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;26:1709–12. doi: 10.1016/0735-1097(95)00394-0. [DOI] [PubMed] [Google Scholar]


