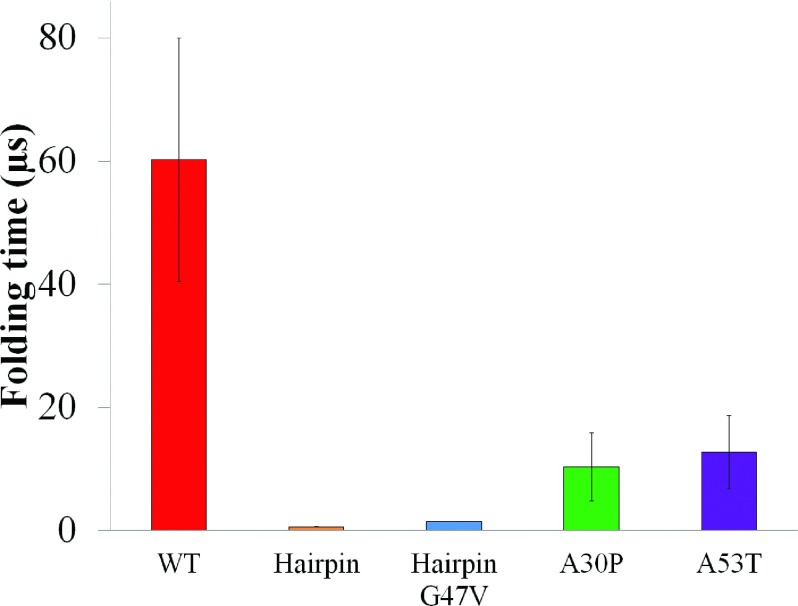
FIG. 4.
Folding times of β-hairpins in region 38-53. The β-hairpin folding for the isolated β-hairpin region or for the A30P and A53T mutants of full-length α-synuclein is faster than that of WT full-length α-synuclein. The time (in μs) needed to achieve a β-hairpin structure in WT full length α-synuclein (Table I, WT), isolated β-hairpin region (hairpin), isolated β-hairpin region with G47V mutation (HairpinG47V), A30P mutant (A30P), and A53T mutant (A53T) of full-length α-synuclein is shown in red, gold, blue, green and purple, respectively. Standard errors are shown as vertical error bars. In the analysis, a folding event is described as a two-state Poisson process arising during independent folding simulations. Formation of β-hairpin arises infrequently in the simulations of α-synuclein, usually not more than once in any simulation. In such case, the β-hairpin formation events can be treated as a two-state Poisson process. The folding times of β-hairpins can thus be estimated by fitting the observed first passage time of folding to Eq. (1) 107 (see Sec. II). In case that folding into a β-hairpin occurs on average more than once in a folding simulation, the folding time was estimated by dividing the accumulated simulation time by the number of times the β-hairpin folds. The folding events were identified according to change of the RMSD with respect to the center structure of the β-hairpin ensemble identified for WT α-synuclein and its A30P, A53T mutants, respectively (Fig. S4).100 Backbone atoms of the β-hairpin region (region 38-53) were selected as reference atoms for alignment and RMSD calculation. A β-hairpin folding event starts with a RMSD value above 5 Å and ends when the RMSD value falls below 3.25 Å (Figs. S4 and S6).100 The folding time of the isolated β-hairpin region is 0.60 ± 0.03 μs, much shorter than the times needed for the same segment to achieve β-hairpin structure in WT full-length α-synuclein (60.23 ± 19.82 μs). The G47V mutation increased the folding time of the isolated β-hairpin region 2.4-fold, namely, to 1.43 ± 0.05 μs. Two mutations of full-length α-synuclein, A30P and A53T, decreased the folding times of WT full-length β-hairpin 5.83- and 4.75-fold to 10.32 ± 5.56 μs and 12.67 ± 5.98 μs.