FIG. 4. Substitution of Glu-210 with Asp or Asn impairs rapid binding and hydrolysis of two ATP molecules by SecA dimer.
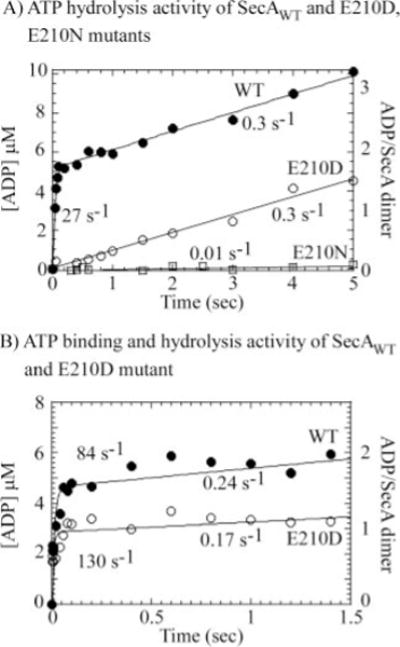
Pre-steady-state acid quench assays were performed with 2.6–3 μM SecA dimer and 500 μM ATP + [α-32P]ATP (final concentrations) at 37 °C. A, wild type (WT) SecA catalyzes rapid ATP hydrolysis (●), with a burst rate constant of 27 ± 2.5 s−1 and amplitude 5.2 ± 0.1 μM (1.8 ATP per dimer), followed by a slow steady-state phase at kcat = 0.3 s−1. In contrast, the SecA-E210N mutant displays no ATP hydrolysis activity in the first catalytic turnover (□). SecA-E210D appears to have residual ATPase activity manifest at a slow linear rate constant of 0.3 s−1 (○). B, pulse-chase analysis reveals rapid binding of 2 ATP molecules to the SecA dimer at an apparent binding rate constant of 0.2 × 106 M−1 s−1 (●; 80–90 s−1 500 M). SecA-E210D also binds ATP rapidly (0.25 × 106 M−1 s−1), but the reduced burst amplitude suggests a defect in ATP binding as well as ATP hydrolysis activity.
