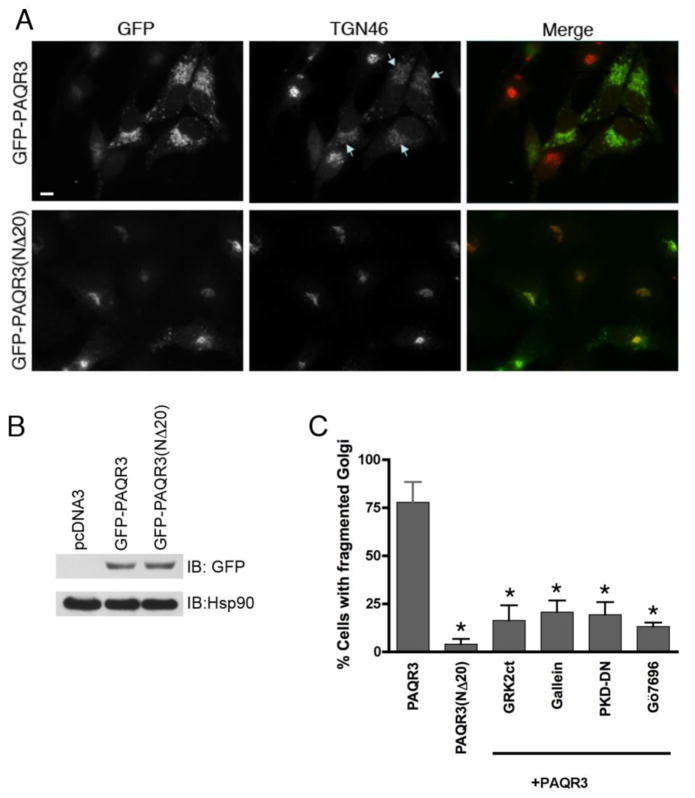
Figure 1. Expression of PAQR3 induces Golgi fragmentation while the Gβ binding-deficient mutant PAQR3(NΔ20) does not fragment the Golgi.
A, HeLa cells were transfected with GFP-PAQR3 (top row) or GFP-PAQR3(NΔ20) (bottom row) for 48 h. Cells were fixed and processed for immunofluorescence microscopy using anti-TGN46 to detect Golgi morphology, and expressed PAQR3 and PAQR3(NΔ20) were detected by intrinsic GFP fluorescence. Arrows indicate Golgi fragmentation. Bar, 10 μm. B, To confirm equivalent expression of GFP-PAQR3 and GFP-PAQR3(NΔ20), immunoblotting was performed using HeLa cell lysates after transfection with pcDNA3, GFP-PAQR3 or GFP-PAQR3(NΔ20). An anti-GFP antibody was used to detect GFP-PAQR3 or GFP-PAQR3(NΔ20) (upper panel), and an anti-Hsp90 antibody was used as a gel loading control (lower panel). Consistent with the immunoblot shown, previous results have demonstrated that PAQR3 and PAQR3(NΔ20) display no detectable difference in mobility [24, 62]. C, Shown is a bar graph quantitating the effect of PAQR3, PAQR3(NΔ20), and the effect of Gβγ and PKD inhibitors in the presence of PAQR3 on Golgi fragmentation. Cells were transfected/treated as described in Figure Legends 1A, 2 and 3. Values are the means +/− S.D. for 3 separate experiments. 100 cells were counted in each experiment. Asterisks indicate statistical significance (p<0.001, t-test) compared to PAQR3 (first bar).