Abstract
Significant innovations in the treatment of patients with multiple sclerosis (MS) have primarily addressed the frequency of flare-ups in relapsing-remitting MS (RRMS). Many advances have been made in this area, and the medical community may be on the verge of a serious discussion of what constitutes a truly effective MS treatment. Certainly, it is important to further delay MS flare-ups and more effectively treat RRMS symptoms. However, great strides in reducing or preventing MS-related disability and providing neuroprotection have been elusive. Many unmet needs are still voiced by patients with MS, clinicians, and caregivers. Current information on the need for progress in various areas is reviewed in this article, including psychosocial care, treatments for progressive MS, biomarker identification, functional outcome measures, individualization of treatment, reducing side effects of medications, and improving medication adherence.
Keywords: multiple sclerosis, relapsing-remitting multiple sclerosis, primary progressive multiple sclerosis, secondary progressive multiple sclerosis, disability, adherence, neuroprotection, biomarkers, outcomes, unmet needs
During the past 15 years, advances in the treatment of relapsing-remitting multiple sclerosis (RRMS) have reshaped the way clinicians and patients approach this disabling chronic disorder. Multiple sclerosis (MS) affects individuals who are in their most productive working and childbearing years1 and is a significant cause of disability. It was estimated that MS affected 400,000 Americans in 2002 (approximately 1 in 85 residents), but this figure has not been updated.2
Pharmaceutical care no longer consists solely of first-generation agents (eg, glatiramer acetate, interferon [IFN] beta-1a and beta-1b). Moreover, the medical community's focus is moving from preventing relapse and reducing its frequency to investigating concepts that minimize MS-related disability.
With several new treatments on the horizon, it appears that providers as well as payers are ready to tackle a critical question: What constitutes a truly effective medication MS management? To answer this question, the term “truly effective” should be examined, considering the outcome expectations of current medications and the ongoing challenges that await patients, providers, and caregivers. This article will review the unmet needs of patients with MS and their providers.
In addition to finding a cure, which, of course, is the greatest priority, the unmet needs are varied and include:
Further delaying progression and developing better treatments for progressive MS
Providing neuroprotection
Delaying or avoiding disability
Reducing active symptoms more effectively
Identifying useful tools and biomarkers
Predicting who would benefit from specific treatments (ie, creating the means for individualizing treatment)
Obtaining better measures of functional outcome
Preventing or ameliorating the adverse effects of current medications
Improving adherence to current medications.
Further Delaying Progression and Developing Better Treatments for Progressive MS
The treatment of patients with MS changed radically when, in the 1990s, modern first-line agents were approved by the US Food and Drug Administration (FDA; eg, the IFNs and glatiramer acetate). According to Markowitz,3 this raised the bar on the definition of effective treatment, and increased the expectations of patients and clinicians. Despite this, clinicians have been able to delay the progression of MS but not eliminate it—a situation that has not changed substantially in 20 years. Regardless, it appears that the definition of treatment effectiveness for MS has been advancing gradually, beyond slowing disease progression to a model of disease remission and neuroprotection.3
To date, patients with progressive MS (primary or secondary) have had few options. However, an investigational agent for the treatment of patients with progressive MS, known as ocrelizumab, is expected to undergo FDA review in 2016.4 In a randomized, double-blind, global, multicenter, phase 3 study of patients with progressive disease, ocrelizumab was associated with less disability than placebo.5
Providing Neuroprotection
The concept of neuroprotection is poorly understood, but it is the focus of much research.3,6 If the neurodegenerative processes of demyelination and axonal deterioration can be slowed or prevented, it may be possible to delay progression and avoid disability.6 The currently available immunomodulating therapies are aimed at preventing ongoing inflammation in the central nervous system, which may provide some neuroprotection. However, this has not been studied adequately, possibly because of the challenges of understanding and measuring neuroprotection at the axonal and neuronal levels.3,7
Relative to the general US population, the median life span of patients with MS is 6 years shorter.8 Unfortunately, it is not known whether neuroprotection might, as a result of decreased neuronal injury and resultant avoidance of disability, extend life expectancy while improving quality of life.
Delaying or Avoiding Disability
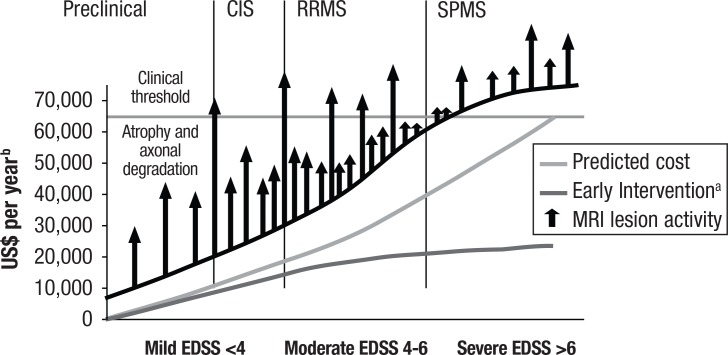
Strober and colleagues9 noted that nearly four-fifths of adults with MS were no longer employed as early as 5 years postdiagnosis, most commonly the result of disability. Based on the typical age range at diagnosis, it is expected that patients will live with the disease for 30 or 40 years. This is clearly a major concern, particularly as patients progress through different stages of disability, including poor mobility and complete dependence on family members or other caregivers for activities of daily living10,11 (Figure 1).
Figure 1. Progression of Disability and Predicted Costs for Multiple Sclerosis.
aCurve is based on an estimation of the decrease in cost for early treatment of about 40% at each range of EDSS.
bDoes not include current drug costs.
CIS indicates clinically isolated syndrome; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Reprinted with permission from Bandari DS, Sternaman D, Chan T, et al. J Manag Care Pharm. 2012;18:S1-S17.
Psychological Disability
MS-related disability can be categorized as physical or psychological (including cognitive). Even though the latter is cited as a huge unmet need by clinicians, researchers, and caregivers (discussed below), particularly because the numerous physical impairments impact mental health as the disease progresses, psychological disability is not being addressed comprehensively by biopharmaceutical manufacturers.
The erosive effects of long-term emotional stress are insidious, but patients with MS generally do not discuss emotional stress with healthcare providers.12 In a 2014 study from Oregon, patients cited lack of time, poor care coordination among providers, overreliance on drug treatments, and inadequate patient self-advocacy as principal reasons for not communicating stress and its effects.12
KEY POINTS
▸ The medical community has begun moving from the singular focus of preventing or reducing disease relapse in patients with MS to minimizing MS-related disability.
▸ With several new treatments on the horizon, providers and payers are now ready to consider what defines a truly effective medication for patients with MS.
▸ The unmet needs of patients with MS include improvements in psychological as well as physical care.
▸ Although the current medications are effective in reducing the frequency of disease relapse, they are also associated with significant adverse effects and adherence challenges.
▸ Additional research on biomarkers, genetic factors, and other factors will be needed to optimize individual therapy and determine which patients have the greatest risk for progression.
In an Italian community–based study of the unmet health and social care needs of more than 1200 patients with MS, 27% of the study population cited psychological support as their greatest unmet need.13 Another study demonstrated that US patients did not usually receive psychological support.14
Caregivers of patients with MS have been referred to as “hidden patients,”13 because patients with MS may require years of focused care by these individuals, particularly when relapse occurs. Care needs are more demanding for patients with progressive disease and severe symptoms.15
During the course of MS, cognitive problems develop in approximately 50% of patients.16,17 These deficits may affect short-term memory, concentration, visuospatial function, executive functions, and processing of information collected through the 5 senses. However, only a small proportion (≤10%) of the population with MS experience cognitive problems severe enough to interfere with daily activities and functioning.16 Memory deficits and impaired abstract or conceptual reasoning appear to be more common in patients with secondary progressive MS (SPMS).17
Physical Disability
Up to one-half of patients who receive disease-modifying therapy for MS do not experience any improvement in mobility.18 In addition to reduced mobility, which is the most common and obvious physical impairment in MS, other problems that occur as the disease progresses include dysphagia and defects in speech and vision; these contribute to worsening scores on the Expanded Disability Status Scale (EDSS), the major tool used to assess clinical status. Currently available medications have only slight to moderate effects in averting the progression of disability (Figure 2).
Figure 2. Disability of Disease-Modifying Therapies for Relapsing-Remitting Multiple Sclerosis Based on Placebo-Controlled, Randomized Trials.
aSignificantly different from placebo.
IFN indicates interferon; IM, intramuscular; SC, subcutaneous.
Source: Bandari DS, Sternaman D, Chan T, et al. J Manag Care Pharm. 2012;18:S1-S17.
In the Italian study mentioned previously, the most cited social need resulting from physical disability was transportation assistance (41%).13 This multivariate analysis showed that patients' unmet health needs were affected by disease stage and degree of disability, but that social care needs were influenced by clinical and sociodemographic factors.13
According to a study from Ireland published in 2015, patients considered physiotherapy their greatest unmet need,19 likely due to the prevalent muscle stiffness and spasms experienced by many patients with advanced disease.
Visual impairment is a leading cause of disability in patients with MS. A European study demonstrated the high importance of vision to patients, who ranked it second to lower-limb function among the most important physical functions affected by MS.20 Not surprisingly, function related to ambulation and mobility was ranked higher than cognitive function in that study. Heesen and colleagues noted that visual function is inadequately represented on the standardized EDSS and the Multiple Sclerosis Functional Composite (MSFC).20 Idiopathic demyelinating optic neuritis, a common type of visual impairment in patients with MS, is a degenerative disorder that can result in temporary unilateral vision loss for a short time, as well as pain that worsens with movement of the affected eye.21
Reducing Active Symptoms More Effectively
Patients who experience systemic MS exacerbations have limited choices to help alleviate the attacks. The principal acute symptoms include muscle stiffness or spasms, general fatigue, depression, and organ problems (eg, bladder control, vestibular dysfunction).22 The effectiveness of symptomatic treatment is variable.23 In most cases, high-dose steroids are administered to blunt the symptoms. For those who need additional treatment (<10% of patients with refractory symptoms), plasmapheresis may be useful.23 Otherwise, treatment is focused on targeting the specific symptoms of the systems affected.
Identifying Useful Tools and Biomarkers
The most common tool to establish the diagnosis of MS and evaluate disease progression is magnetic resonance imaging (MRI). T1- and T2-weighted images can help determine “lesion load” and may be useful for predicting future progression and disability. A 1999 study demonstrated that if lesion load is measured very early in the course of MS, it may predict the relapse rate.24 However, the value of MRI as a biomarker for anything other than lesion load or inflammation may be limited.25
Many other possible tools and biomarkers for diagnosing MS and monitoring its course have been described, including immunogenetic, laboratory, and imaging signs. (Table 1 of the article by Katsavos and Anagnostouli7 contains a comprehensive list of potential biomarkers.) However, the utility of biomarkers thought to be good targets is hampered by lack of specificity, as well as expense and difficult challenges for use in daily practice.7 There are biomarkers for therapeutic response (eg, expression levels of unblocked alpha-4 integrin on peripheral mononuclear blood cells indicate natalizumab efficacy), as well as biomarkers to determine whether the disease is transitioning to a more progressive stage (eg, vascular endothelial growth factor–alpha may predict progression from RRMS to SPMS).7
Table.
Multiple Sclerosis Outcome Measures
| 12-Item MS Walking Scale 6-minute walk test 9-hole peg test Activities-Specific Balance Confidence Scale Berg balance test Box and blocks test Dizziness Handicap Inventory Dynamic Gait Index Fatigue Scale for Motor & Cognitive Functions Functional Assessment of MS Functional Independence Measure Functional reach Goal Attainment Scale Guy's Neurologic Disability Scale Minimal Inspiratory/Expiratory Pressure Maximum Oxygen Uptake (VO2 max & VO2 peak) Modified Fatigue Impact Scale Multiple Sclerosis Functional Composite Multiple Sclerosis Impact Scale (MSIS-29) Multiple Sclerosis Quality of Life (MS-QoL 54) Multiple Sclerosis Quality of Life Inventory Rivermead Mobility Index Timed 25-foot walk Timed Up & Go (TUG) cognitive and manual test Trunk Impairment Scale Visual Analog Scale (for fatigue) 36-Item Short Form Health Survey (SF-36) of the Medical Outcomes Study |
Source: Neurology Section of the American Physical Therapy Association. MS-EDGE Outcomes Measures for Research. 2012. www.neuropt.org/docs/degenerative-diseases-sig/ms-edge_research_recs60F4A42650E1.pdf?sfvrsn=2.
The cerebrospinal fluid (CSF) has been an interesting target for biomarker research in MS. The CSF proteome, or the proteins that are expressed overall by a body, is a fertile area of such research.7,26 Ongoing work entails comparing CSF proteome levels between healthy individuals and patients with MS to identify which proteins may be the most promising biomarkers.26
Also hampering the effort to establish suitable biomarkers for MS risk, diagnosis, and progression is the lack of understanding of the etiology of this disease.7 Until such understanding occurs, clinicians may be limited to treating MS rather than preventing it.
Progress has been made in using biomarkers to help make therapy safer. An anti-JCV (John Cunningham polyomavirus) antibody test, approved by the FDA in 2012,27 has helped lower the risk of lethal progressive multifocal leukoencephalopathy (PML) in patients taking natalizumab, enabling its use as a first-line agent in those with a negative test result; however, false-negative results can occur.28,29 Although testing for JCV antibodies has helped reduce the risk of PML substantially, this serious adverse effect cannot be eliminated entirely.28
Predicting Who Would Benefit from : Specific Treatments
For patients recently diagnosed with MS, the choice of disease-modifying treatment is not necessarily evidence-based; such decision-making often is influenced by provider preference, adherence rates, and perceptions of the risk–benefit profiles of each drug.30 Only recently has useful information been generated on comparative effectiveness of classes. However, the effectiveness of any agent varies from patient to patient, and MRI evidence of lesion progression continues in most patients despite optimal therapy.6
Although a biomarker (antibody presence/absence) exists for determining whether patients taking natalizumab will be at risk for PML, there is no available biomarker for predicting whether MS will respond to this agent. Another unmet need is rapid prediction of whether a particular drug, which may be an expensive specialty product, will produce a response. Therapeutic trials of MS drugs may last 6 months or more.26 This contributes to high costs as well as delays in finding optimal treatment for an individual patient, potentially leading to additional disability and relapse in the interim.
More information on the profile of each patient's clinical phenotype and biomarkers may help determine additional subtypes of MS and predict who may be most likely to benefit from specific specialty treatments.31
Obtaining Better Measures of Functional Outcome
Because MS affects several areas of physical and neurologic function, no single measure (such as walking distance in a specified time) is adequate to assess symptomatic improvement or deterioration. A task force of neurologic physical therapists evaluated 63 possible outcome measures covering body structure and function, activity, and participation, and recommended that 27 outcome measures be used for research (Table).32 It was concluded that these measures have good psychometric properties and good clinical utility.
Clinicians who treat patients with MS are aware of the limitations of neurologic examination, yet this type of examination is the foundation for the EDSS.33 Moreover, a patient's current level of performance or functioning may fluctuate, and may not directly reflect the disease pathology.33
In 2012, the International Advisory Committee on Clinical Trials in Multiple Sclerosis stated that “many of the available disability outcome measures used in clinical trials of multiple sclerosis are insensitive to change over time, inadequately validated, or insensitive to patient-perceived health status or quality of life.”34 They recommended that, as a result of the changing emphasis on treatments that may directly affect disability in MS, new measurement tools may be needed to adequately address this matter. However, the committee members discouraged making radical changes to the EDSS because of its current level of acceptance by regulators. Instead, they recommended refinements to the MSFC and its component measures, and supported greater use of other composite end-point measures that incorporate patient-reported outcomes and the adjunctive use of biomarkers for disability assessment. Validation remains a key challenge.34
Preventing or Ameliorating Adverse Effects of Current Medications
The disease-modifying therapies for MS that have been approved for treatment include the older first-generation agents (injectable IFN-beta, glatiramer acetate), oral drugs (dalfampridine, dimethyl fumarate, fingolimod, teriflunomide), monoclonal antibodies (alemtuzumab, natalizumab), and an immunosuppressive agent (mitoxantrone).
As discussed above, natalizumab is associated with PML, particularly if the drug is used for more than 2 years.9 Another risk factor for PML is previous use of immunologic agents. Natalizumab also has been associated with hepatic enzyme abnormalities, infections, and postinfusion reactions.11 Mitoxantrone has been linked to cardiac abnormalities.11
A serious adverse event associated with teriflunomide is the potential for fetal malformation,11,28 which is emphasized by the black box warning about the parent compound (leflunomide).28 Teriflunomide also is associated with liver function abnormalities, diarrhea, nausea, flulike symptoms, and hair loss.9,31
The oral medication fingolimod has been linked to various adverse events, including bradycardia and atrioventricular block following the first dose, flulike symptoms, lymphopenia, macular edema, and abnormal elevation of liver enzymes. Like teriflunomide, fingolimod also may cause fetal malformation.11,28
Patients treated with alemtuzumab are subject to transient gastrointestinal symptoms and opportunistic infections. Moreover, they may have a higher-than-normal risk of hyperthyroidism or immune thrombocytopenic purpura because of alemtuzumab's mechanism of action: inhibiting T-cells and B-cells for prolonged periods.35
In contrast, dimethyl fumarate, which is deemed more effective than teriflunomide,28 may also have a better side effect profile. The most common side effects of dimethyl fumarate are gastrointestinal problems and flushing, both of which are considered transient in nature.28 However, its prescribing information also includes warnings about PML and lymphopenia.36
Overall, long-term safety data are lacking for oral medications for MS, including the most recent additions to the class.10,31,37 This has been a concern of many clinicians and investigators, especially because of the lengthy duration of the disease (several decades or more).38
Improving Adherence to Current Medications
Although disease-modifying medications are effective when used early in RRMS, treatment adherence has been challenging and may affect patient outcomes. As noted in a review of the literature, some adherence difficulties are associated with traditional medications administered by injection.39 The adherence rates for injectable disease-modifying therapies range from 41% to 88%, with the lower rates associated with retrospective studies.39
Even with pretreatment counseling and education on the expectations and potential side effects of therapy, a study showed that 17% of patients with MS stopped taking their medications. Therapy discontinuation was more common for patients with SPMS (30%) than for those with RRMS (13%).10
Complicating this issue is the high patient cost-sharing for the medications most often used for patients with MS, and the fact that neurologists tend to overestimate their patients' adherence.9 Although oral therapy is usually preferred by patients, there is little evidence indicating that oral drugs for MS are associated with better treatment adherence or persistence than the injectable agents. Despite general evidence that specialty pharmacy programs can improve adherence,40 there is little information to suggest that this is true for patients with MS.11
Conclusion
Despite the attention afforded MS in recent years, many unmet needs remain in terms of therapeutics, disability avoidance, and outcome measures. More progress is needed not only in basic science and genetic and other factors, but in understanding patients' priorities, which may improve (and possibly extend) patients' lives, optimizing their ability to be productive. Further attention also must be given to managing the costs associated with treatment.
Author Disclosure Statement
Mr Mehr and Ms Zimmerman have no conflicts of interest to report.
Contributor Information
Stanton R. Mehr, President, SM Health Communications, Newtown, PA
Marj P. Zimmerman, President, RxDirections, Bucyrus, KS.
References
- 1.National Multiple Sclerosis Society. Who gets MS? 2015. www.nationalmssociety.org/What-is-MS/Who-Gets-MS. Accessed September 17, 2015.
- 2.National Multiple Sclerosis Society. Estimating the prevalence of MS. 2015. www.nationalmssociety.org/About-the-Society/MS-Prevalence. Accessed September 17, 2015.
- 3.Markowitz CE. The current landscape and unmet needs in multiple sclerosis. Am J Manag Care. 2010; 16:S211–S218. [PubMed] [Google Scholar]
- 4.Hirschler B. Roche gets jump on rivals in race for progressive multiple sclerosis treatment. September 28, 2015. www.reuters.com/article/2015/09/28/us-roche-multiple-sclerosis-rivals-idUSKCN0RS1M720150928. Accessed September 28, 2015.
- 5.Silva P. Could Genentech's ocrelizumab become the first effective primary progressive MS therapy? Multiple Sclerosis News Today. September 29, 2015. http://multiplesclerosisnewstoday.com/2015/09/29/genentechs-ocrelizumab-become-first-effective-primary-progressive-ms-therapy/. Accessed September 29, 2015.
- 6.Katz Sand IB, Krieger S. Emerging strategies for the treatment of multiple sclerosis. Future Neurol. 2012; 7:193–207. [Google Scholar]
- 7.Katsavos S, Anagnostouli M. Biomarkers in multiple sclerosis: an up-to-date overview. Mult Scler Int. 2013; 2013:340508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman DW, Reshef S, Golub HL, et al. Survival in commercially insured multiple sclerosis patients and comparator subjects in the U.S. Mult Scler Relat Disord. 2014; 3:364–371. [DOI] [PubMed] [Google Scholar]
- 9.Strober LB, Christodoulou C, Benedict RH, et al. Unemployment in multiple sclerosis: the contribution of personality and disease. Mult Scler. 2012; 18:647–653. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SS, Philbrick AM. Improving multiple sclerosis care: an analysis of the necessity for medication therapy management services among the patient population. J Manag Care Spec Pharm. 2014; 20:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandari DS, Sternaman D, Chan T, et al. Evaluating risks, costs, and benefits of new and emerging therapies to optimize outcomes in multiple sclerosis. J Manag Care Pharm. 2012; 18:S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senders A, Sando K, Wahbeh H, et al. Managing psychological stress in the multiple sclerosis medical visit: patient perspectives and unmet needs. J Health Psychol. 2014. Dec 19. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 13.Ponzio M, Tacchino A, Zaratin P, et al. Unmet care needs of people with a neurological chronic disease: a cross-sectional study in Italy on multiple sclerosis. Eur J Public Health. 2015; 25:775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minden SL, Ding L, Cleary PD, et al. Improving the quality of mental health care in multiple sclerosis. J Neurol Sci. 2013; 335:42–47. [DOI] [PubMed] [Google Scholar]
- 15.Golla H, Mammeas S, Galushko M, et al. Unmet needs of caregivers of severely affected multiple sclerosis patients: a qualitative study. Palliat Support Care. 2015:1–9. [DOI] [PubMed]
- 16.National Multiple Sclerosis Society. Cognitive changes. 2015. www.nationalmssociety.org/Symptoms-Diagnosis/MS-Symptoms/Cognitive-Changes. Accessed September 24, 2015.
- 17.Grazioli E, Yeh AE, Benedict RHB, et al. Cognitive dysfunction in MS: bridging the gap between neurocognitive deficits, neuropsychological batteries and MRI. Future Neurol. 2008; 3:49–59. [Google Scholar]
- 18.Berger JR. Functional improvement and symptom management in multiple sclerosis: clinical efficacy of current therapies. Am J Manag Care. 2011; 17(suppl 5):S146–S153. [PubMed] [Google Scholar]
- 19.Lonergan R, Kinsella K, Fitzpatrick P, et al. Unmet needs of multiple sclerosis patients in the community. Mult Scler Relat Disord. 2015; 4:144–150. [DOI] [PubMed] [Google Scholar]
- 20.Heesen C, Böhm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008; 14:988–991. [DOI] [PubMed] [Google Scholar]
- 21.Galetta SL, Villoslada P, Levin N, et al. Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm. 2015; 2:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Multiple Sclerosis Society. MS symptoms. 2015. www.nationalmssociety.org/Symptoms-Diagnosis/MS-Symptoms. Accessed October 23, 2015.
- 23.National Multiple Sclerosis Society. Medications. www.nationalmssociety.org/Treating-MS/Medications#section-2. Accessed October 23, 2015.
- 24.Kappos L, Moeri D, Radue EW, et al. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet. 1999; 353:964–969. [DOI] [PubMed] [Google Scholar]
- 25.Foley JF, Zerkowski K, Nair KV. Evidence for long-term use of intramuscular interferon beta-1a: an overview of relapse, disability, and MRI data from selected clinical trials. J Manag Care Pharm. 2013; 19(suppl A):S4–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilner AN, Teunissen CE. MS biomarkers in the clinic: around the corner? December 20, 2011. www.medscape.com/viewarticle/755699. Accessed September 10, 2015.
- 27.US Food and Drug Administration. FDA permits marketing of first test for risk of rare brain infection in some people treated with Tysabri. January 20, 2012. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm288471.htm. Accessed October 23, 2015.
- 28.Jeffery DR. Recent advances in treating multiple sclerosis: efficacy, risks and place in therapy. Ther Adv Chronic Dis. 2013; 4:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozic C, Richman S, Plavina T, et al. Anti-John Cunningham virus antibody prevalence in multiple sclerosis patients: baseline results of STRATIFY-1. Ann Neurol. 2011; 70:742–750. [DOI] [PubMed] [Google Scholar]
- 30.Owens GM, Olvey EL, Skrepnek GH, Pill MW. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost-benefits of disease-modifying therapies. J Manag Care Pharm. 2013; 19(suppl A):S41–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanasescu R, Ionete C, Chou IJ, Constantinescu CS. Advances in the treatment of relapsing-remitting multiple sclerosis. Biomed J. 2014; 37:41–49. [DOI] [PubMed] [Google Scholar]
- 32.MS-EDGE Outcomes Measures for Research. Neurology Section of the American Physical Therapy Association 2012. www.neuropt.org/docs/degenerative-diseases-sig/ms-edge_research_recs60F4A42650E1.pdf?sfvrsn=2. Accessed September 28, 2015.
- 33.Bermel R, Waldman A, Mowry EM. Outcome measures in multiple sclerosis. Mult Scler Int. 2014; 2014:439375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen JA, Reingold SC, Polman CH, Wolinsky JS; International Advisory Committee on Clinical Trials in Multiple Sclerosis. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol. 2012; 11:467–476. [DOI] [PubMed] [Google Scholar]
- 35.Sanofi. Lemtrada prescribing information. November 2014. http://products.sanofi.us/lemtrada/lemtrada.pdf. Accessed September 30, 2015.
- 36.Biogen Idec. Tecfidera prescribing information. April 2015. www.tecfidera.com/pdfs/full-prescribing-info.pdf. Accessed September 30, 2015.
- 37.Noyes K, Weinstock-Guttman B. Impact of diagnosis and early treatment on the course of multiple sclerosis. Am J Manag Care. 2013; 19(suppl):S321–S331. [PubMed] [Google Scholar]
- 38.Tramacere I, Del Giovane C, Salanti G, et al. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2015; 9:CD011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstock-Guttman B. An update on new and emerging therapies for relapsing-remitting multiple sclerosis. Am J Manag Care. 2013; 19:S343–S354. [PubMed] [Google Scholar]
- 40.Visaria J, Henderson R, Glave Frazee S. Specialty pharmacy improves adherence to imatinib. Am J Pharm Benefits. 2013; 5(special issue):SP33–SP39. [Google Scholar]