Practical Implications
Chronic traumatic encephalopathy should be considered in older patients with a history of remote or indirect head trauma and late-life dementia, with atypical clinical features serving as a useful clue.
First described as dementia pugilistica in boxers, chronic traumatic encephalopathy (CTE) is associated with repeated head injury and has received much attention after several high-profile National Football League cases.1 Recent work suggests that both the nature of potential predisposing trauma and the clinical spectrum of CTE may be broader than previously recognized.1–3 We report a case of CTE presenting as Alzheimer-like dementia in a war reporter with a history of prolonged proximity to shelling but no acute head trauma.
Case description
A 77-year-old retired war journalist presented to our clinic with cognitive and memory complaints. Seven years prior he had developed apathy and navigational difficulties, initially attributed to recent leuprolide therapy for prostate cancer. In the following years, he had a slow progression of short-term memory deficits, anxiety, impulsivity in managing his finances, and increased alcohol intake. He was diagnosed with clinically probable Alzheimer disease (AD) at an outside clinic and started on donepezil, memantine, and escitalopram at age 75, which his wife felt provided substantial benefit.
At the time he presented, he met criteria for enrollment in clinical trials of anti-amyloid therapy for AD4 and received bapineuzamab. He had stopped driving due to navigational difficulties, needed prompting to wash and groom himself, and assisted with only simple household tasks. He had begun craving sweet foods. In his early 80s, he began to tell stories repeatedly and wake disoriented late at night. He died at age 84 after a myocardial infarction.
While covering one conflict, he lost most of his hearing on one side from proximity to shelling and mortar fire, with residual tinnitus. He had no concussion, head injury, loss of consciousness, or chemical exposures per his wife. He had a single car accident in his 30s wherein he rear-ended another car; he had no loss of consciousness or residual symptoms. His medical history was noncontributory. His mother, 2 sisters, and a paternal cousin had been diagnosed with late-onset AD.
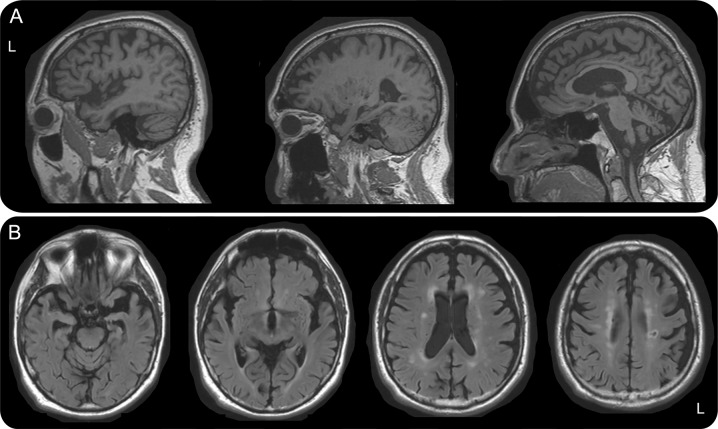
At age 77, neurologic examination showed only mild right-sided bradykinesia and retropulsion. Neuropsychological testing revealed difficulties with executive function, visual more than verbal memory, and spatial reasoning. Imaging showed predominantly left-sided temporal and bilateral dorsal frontoparietal atrophy (figure 1). Genetic testing showed APOE status of ε2/ε4.
Figure 1. MRI of the brain showing left-sided temporal and bilateral dorsal frontoparietal atrophy.
Sagittal magnetization-prepared rapid gradient-echo (A) and axial fluid-attenuated inversion recovery (B) images demonstrate predominantly left-sided atrophy of the temporal poles, mesial temporal lobes, and insulae with more moderate generalized dorsal temporoparietal atrophy, which is relatively symmetric. There is a moderate amount of white matter disease in the centrum semiovale in a typical vascular distribution as well as a small lacune in the left posterior frontal white matter.
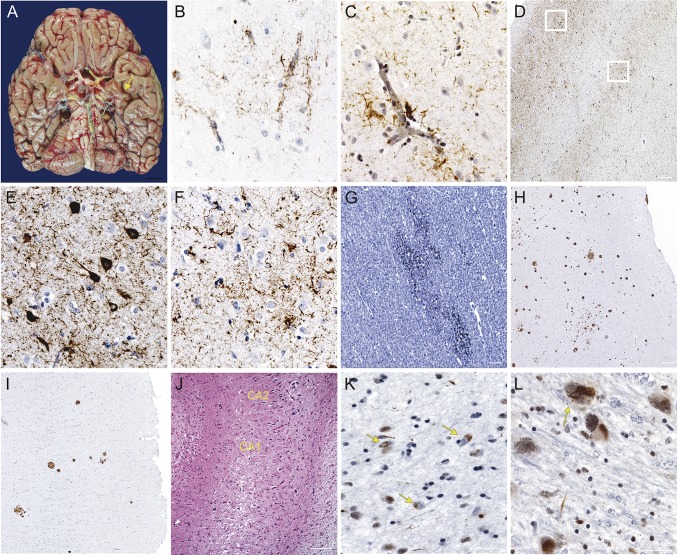
Postmortem assessment was consistent with CTE, showing tau deposition concentrated in superficial layers of cortex, with highest density in inferior temporal gyrus, amygdala, hippocampus, and insula. In areas of milder tau deposition (e.g., angular gyrus), tau was seen specifically in the depths of sulci. TAR DNA-binding protein 43 (TDP-43) inclusions were seen in a similar distribution. Neuritic plaques were frequent in frontal and anterior temporal cortices but scarce in posterior cortices. Diffuse axonal injury was seen in the white matter, particularly frontally (figure 2). AD pathologic changes were graded as intermediate (A1, B2, C3)5; there was no α-synuclein pathology.
Figure 2. Postmortem assessment consistent with CTE.
(A) Inferior view of the brain (fresh weight: 1,237 g). Note the atrophy of mesial temporal structures, greater on the left (arrow). Despite severe atherosclerosis of the circle of Willis, the brain was free of macroscopic vascular injuries. No signs of chronic hemorrhage were seen. (B–F) Immunohistochemistry against phosphorylated tau (p-tau) (CP-13, 1:500, gift of Peter Davies, NY). P-tau deposition surrounding small vessels was conspicuous, as seen in the anterior cingulate cortex (B) and inferior temporal gyrus (C), and suggestive of chronic traumatic encephalopathy (CTE). (D) The inferior temporal gyrus showed abundant tau deposition in neurons and glia. However, atypically for Alzheimer disease (AD), neurofibrillary tangles were concentrated in the superficial layers (E), with largely glial pathology and treads populating cortical layers V and VI (F). Tau pathology was abundant in frontal and anterior temporal cortices and brainstem nuclei, whereas posterior temporal, parietal, and occipital cortices were largely devoid of tau inclusions. (G–I) Immunohistochemistry against β-amyloid (4G8, 1:5,000, Signet Pathology Systems, Dedham, MA). Diffuse axonal injury was seen in subcortical white matter, including the anterior cingulate cortex (G). This is classically seen in acute traumatic brain injury but can linger in some cases of CTE. Frequent neuritic plaques populated the medial frontal gyrus (H) and other frontal and anterior temporal cortices, but scant plaques, at most, were seen in angular gyrus (I), superior temporal gyrus, and striate cortex. This regional plaque distribution is atypical for AD and has not been observed after treatment with β-amyloid–lowering therapy. (J) Hippocampus stained for hematoxylin and eosin. Note the abrupt loss of neurons between CA2 and CA1, characteristic of hippocampal sclerosis. (K, L) Immunohistochemistry against TAR DNA-binding protein 43 (TDP-43) (1:500, Proteintech, Chicago, IL). TDP-43 inclusions were found in inferior temporal gyrus, amygdala (K), hippocampal formation, and midbrain. These inclusions were mostly cytoplasmatic (arrows in K) and assumed a round or crescent shape but were granular at times. (L) Substantia nigra showing TDP-43 inclusions. The arrow points to a skein-like inclusion. TDP-43 and tau pathology in the substantia nigra could explain the parkinsonism in this case despite the lack of α-synuclein pathology. Scale bars: A: 1 cm; B, C, D, H, I: 100 μm; C, E, F, L: 10 μm; G, J, K: 50 μm.
DISCUSSION
Here we present a case of an older man with clinical AD with atypical features evolving late in his illness and underlying primary CTE. CTE is associated with repeated head trauma in athletic, military, and other settings.1 Most cases feature personality disturbances followed by cognitive deficits and parkinsonism, but CTE may have a predominantly cognitive presentation, especially in older individuals.6 Evidence suggests that different forms of head trauma, including blast exposure, can result in CTE3; this is the first case without associated direct head trauma in our group's experience and highlights the importance of considering CTE in similar circumstances. This case broadens the clinical phenotype of CTE: the patient was asymptomatic for 35 years following his closest proximity to shelling and presented with a late-onset AD phenotype. Notably, his illness progressed more slowly than expected for AD, and he had late atypical features such as sweet cravings and poor hygiene characteristic of frontotemporal cortical involvement.
A characteristic tauopathy is found in all CTE cases.7 AD-type pathology could partially explain our patient's dementia, although the pattern of neuritic plaque burden, with heavier deposition frontally and mild to no deposition posteriorly, is unusual. In fact, recent work raises the question of whether CTE is better described as a polyproteinopathy, including TDP-43 and β-amyloid deposition, especially in older patients with a longer interval between traumatic brain injury and death.1,2 The atypical β-amyloid distribution could be a consequence of bapineuzumab therapy. However, regional amyloid cleaning has yet to be reported in similar cases.4
AUTHOR CONTRIBUTIONS
Carolyn A. Fredericks, MD: conceptualization of the case report, case analysis and interpretation, drafting and revision of the manuscript for intellectual content. Mary Koestler, RN, PhD: clinical care of the patient, case analysis and interpretation, revision of the manuscript for intellectual content. William Seeley, MD: postmortem analysis of the case and interpretation, revision of the manuscript for intellectual content. Bruce Miller, MD: conceptualization of the case report, case analysis and interpretation, revision of the manuscript for intellectual content. Adam Boxer, MD, PhD: clinical care of the patient, postmortem analysis of the case and interpretation, revision of the manuscript for intellectual content. Lea T. Grinberg, MD, PhD: postmortem analysis of the case and interpretation, conceptualization of the case report, case analysis and interpretation, drafting and revision of the manuscript for intellectual content.
STUDY FUNDING
Institutional support from NIH grants P50AG023501, P01AG019724.
DISCLOSURES
C.A. Fredericks receives research support from NIH. M. Koestler reports no disclosures. W. Seeley serves on a scientific advisory board for Biogen Idec; serves on the editorial boards of Annals of Neurology and Acta Neuropathologica; serves as a consultant for Summer Street Research Partners; and receives research support from NIH/NIA, The John Douglas French Alzheimer's Disease Foundation, Consortium for Frontotemporal Dementia Research, Tau Consortium, John D. and Catherine T. MacArthur Foundation, Alzheimer's Disease Drug Foundation, and Association for Frontotemporal Dementia. B. Miller serves on scientific advisory boards for Tau Consortium, The John Douglas French Foundation, The Larry Hillblom Foundation, NIHR in Dementia (UK), FasterCures: A Center of the Milken Institute, and Tangled Bank Studios; serves on the editorial boards of Neurocase, Cambridge University Press, and Guilford Publications, Inc.; receives publishing royalties for Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008); and receives research support from the NIH/NIA and CMS. A. Boxer serves on a scientific advisory board for Asceneuron; has received funding for travel from the International Society for CNS Clinical Trials Methodology and the Movement Disorders Society; serves as a consultant for Ipierian, Isis, and Merck; and receives research support from Avid, BMS, C2N, Cortice, Forum, Genentech, Janssen, Pfizer, Eli Lilly, TauRx, NIH, The Tau Research Consortium, the Bluefield Project to Cure Frontotemporal Dementia, and the Alzheimer's Association. L.T. Grinberg has received funding for travel from connectmed and speaker honoraria from Israeli Neurological Association; serves as an Associate Editor for Frontiers in Dementia and Cell and Tissue Banking; serves as a consultant for AVID Radiopharmaceuticals; and receives research support from the NIH/NIA, The John Douglas French Alzheimer's Foundation, Alzheimer's Association, and Rainwater Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol 2015;25:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013;9:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012;4:134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salloway S, Sperling R, Fox NC, et al. ; Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med 2014;370:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montine TJ, Phelps CH, Beach TG, et al. ; National Institution on Aging; Alzheimer's Association. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med 1973;3:270–303. [DOI] [PubMed] [Google Scholar]