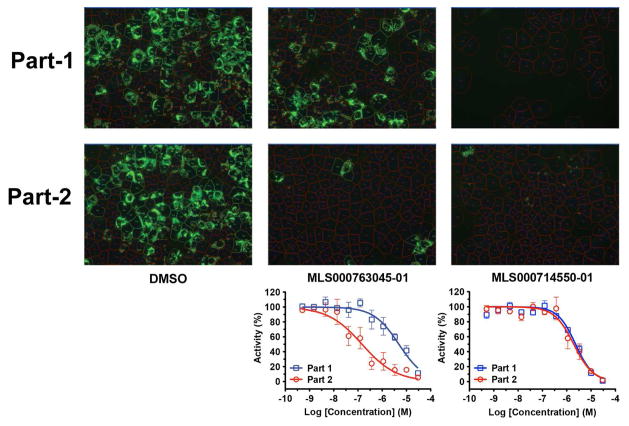
Figure 3.
Hits validation with 2-part HCV infection and core immunofluorescence staining assay. Naïve Huh7.5.1 cells in 384-well plates were infected with wild-type HCVcc at 0.05 moi. Compounds in 1:3 serial dilutions starting at 30 μM were administered to the cells at the time of infection (part 1). After 48 h of culture, the media were transferred to a fresh plate of cells (part 2). Percentages of HCV core-positive cells were calculated and normalized against the DMSO control and plotted as CRCs. Two representatives of compounds targeting at early and late stages respectively are shown.