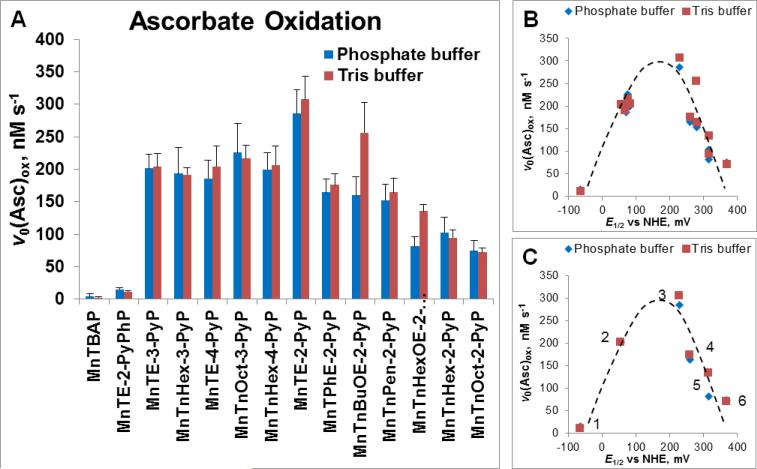
Figure 3. Ability of MnPs to catalyze ascorbate oxidation as assessed spectrophotometrically via determination of initial rates of ascorbate oxidation, v0 (Asc)ox.
(A) The v0(Asc)ox was measured for various MnPs which differ vastly with respect to their redox properties, lipophilicity/bioavailability, polarity, size and bulkiness. Initial rates were determined with 5 μM MnP, 5 mM EDTA and 0.15 mM sodium ascorbate under aerobic conditions at (25 ± 1)0C and at pH 7.8 maintained with either 0.05 M potassium phosphate or Tris buffer. (B) Relationship between v0(Asc)ox and E1/2 is of bell-shape. (C) Six cationic MnPs with the E1/2 ranging from −65 to +340 mV vs NHE are plotted: (#1) MnTE-2-PyPhP5+, (#2) MnTE-3-PyP5+, (#3) MnTE-2-PyP5+, (#4) MnTPhE-2-PyP5+, (#5), MnTnHexOE-2-PyP5+, and (#6) MnTnOct-2-PyP5+.