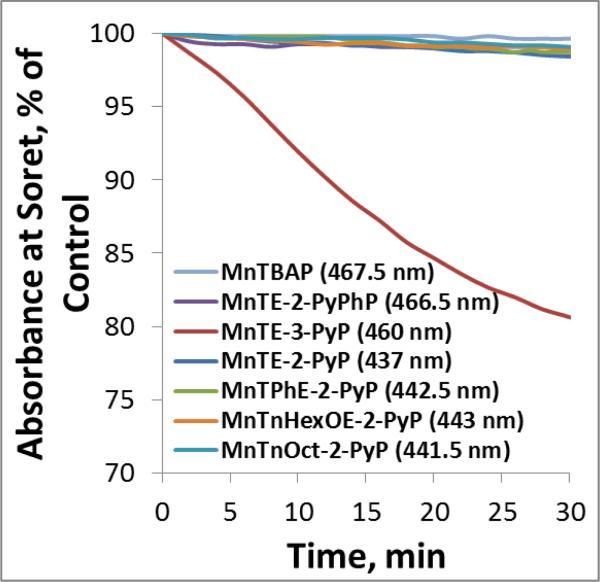
Figure 7. Stabilities of MnPs towards Asc-mediated oxidative degradation.
5 μM MnPs were incubated with 1 mM ascorbate in the 0.05 M Tris buffer at pH 7.8. The H2O2, which is produced during the MnP/Asc cycling, accumulates in the solution and leads to MnP degradation The stability of MnPs was followed spectrophotometrically at Soret band which corresponds either to Mn +3 (MnIIITE-3-PyP5+ and MnIITE-2-PyPhP4+), or +2 oxidation state (MnIITE-2-PyP4+, MnTnOct-2-PyP5+, MnTnHexOE-2-PyP5+ MnIITPhE-2-PyP4+) oxidation states. MnIIITE-3-PyP5+ is hardly reducible under aerobic conditions and once reduced re-oxidizes to Mn +3 oxidation state order of magnitude faster than MnTE-2-PyP5+ [33].